Figure 4. Structure of the anti-termination complex.
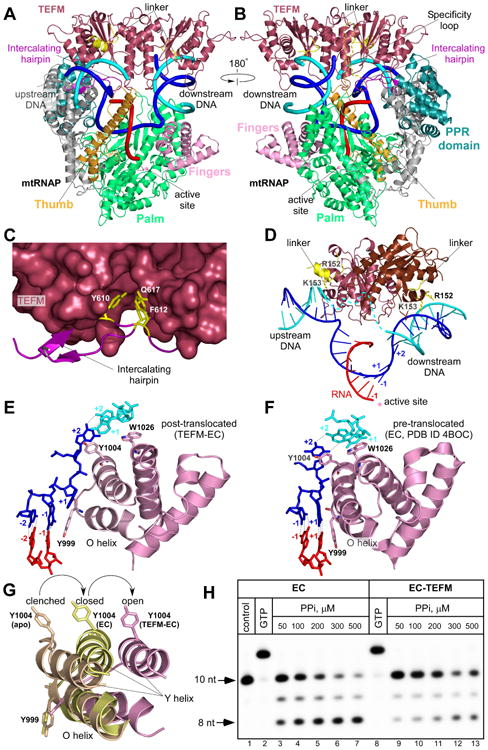
(A,B) TEFM binding to the EC. MtRNAP (ribbon model) is shown with the major domains colored: Thumb: bright orange; Palm: lime-green; NTD: grey; PPR: teal; Fingers domain: pink. RNA is shown in red, template DNA strand in blue, non-template DNA strand in cyan. Intercalating hairpin and specificity loop (modeled) of mtRNAP are highlighted in purple and yelloworange, respectively.
(C). Close-up view of the TEFM binding site at the intercalating hairpin of mtRNAP. TEFM is shown as surface representation. Conserved residues (yellow) in mtRNAP are shown as sticks.
(D) TEFM interacts with the RNA-DNA scaffold in the EC. TEFM (ribbon representation) binds at junctions of the RNA-DNA hybrid and interacts with downstream and upstream DNA. The +1 template DNA base is not paired to its non-template counterpart, indicating the post-translocated conformation state of the EC. Note the proximity of the inter-domain linkers (residues 149-159, yellow) to the DNA duplexes; positively charged residues implicated in interaction with DNA are shown as sticks.
(E,F) Orientation of the O/Y helices of the fingers domain of mtRNAP relative to the 3′ end of RNA in the post-translocated (E) and pre-translocated (F, PDB ID 4BOC) ECs.
(G) Movement of the O/Y helixes in the fingers domain of mtRNAP accompanies formation of the EC and RNA translocation. MtRNAPs from the apo form (PDB ID 3SPA), pre-translocated EC (PDB ID 4BOC) and post-translocated EC-TEFM (this work) were superimposed using the conserved palm domain (residues 643-1230).
(H) TEFM stabilizes the post-translocated state of the EC. A pyrophosphorolytic assay was performed using the EC (lanes 3-7) and the EC-TEFM complex (lanes 9-13). The control (lane 1) is the EC. The assembled complexes were nearly 100% active as evident by efficient extension of the RNA primer by GTP (lanes 2,8). See also Table S2, Figure S3, S4
