Fig. 1.
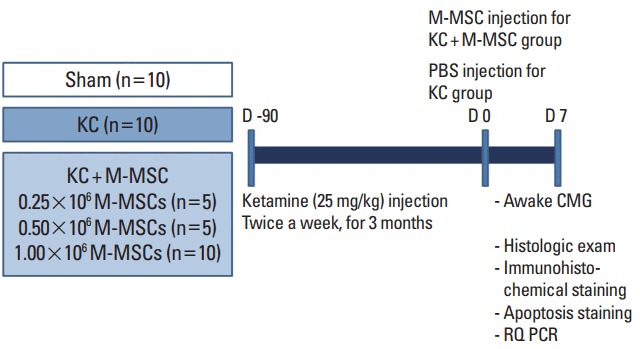
Schematic diagram of the main study design. The control group (KC group) and the experimental group (KC+M-MSC group) were given ketamine twice weekly for 12 weeks. Interventions involved a single administration of human embryonic stem cell-derived multipotent mesenchymal stem cells (M-MSCs) at the indicated doses (0.25, 0.5, and 1×106 cells). One week after M-MSC injection, therapeutic outcomes were evaluated. KC, ketamine-induced cystitis; M-MSC, multipotent mesenchymal stem cell; PBS, phosphate buffered saline: CMG, cystometrography; RQ PCR, real-time quantitative polymerase chain reaction.
