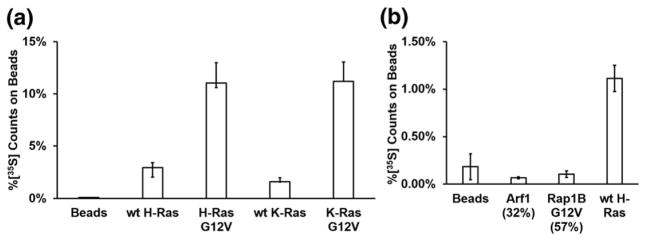
Fig. 3.
Binding of RasIn1 to different Ras homologs. (a) Radiolabeled RasIn1 shows excellent binding to different versions of Ras (wild-type K- or H-Ras, and G12V mutant K- or H-Ras), showing that the recognition of the active Ras state is robust. (b) Binding specificity of RasIn1 against homologous members of the Ras superfamily. RasIn1 binds specifically to active K-Ras, but less to homologous Ras family members Rap1B(G12V) (p = 0.01) or Arf1 (0.02) (percentage of sequence identity to the K-Ras G domain is shown in parentheses). Error bars indicate the standard deviation of the mean.