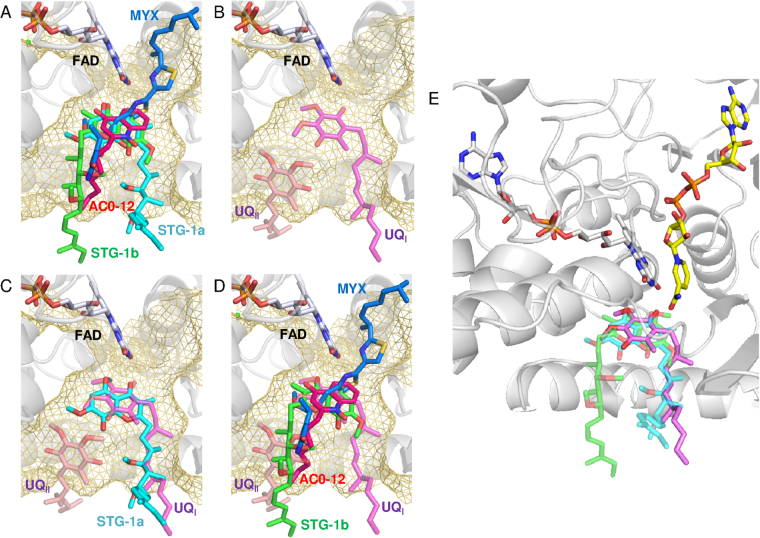
Figure 6.
A comparison of the binding of stigmatellin, AC0-12 and myxothiazol to Ndi1, in relation to the UQI and UQII sites. (A) Close-up views of the overlap projection of the binding of stigmatellin (STG-1a: aqua; STG-1b: green), myxothiazol (blue) and AC0-12 (red) to Ndi1 (transparent gray). (B) A close-up view of the ubiquinone at the UQI (transparent pink) and UQII (transparent pale orange) sites, which were adapted by superimposing the Ndi1-ubiquinone complex structure (PDB code: 4G74) proposed by Feng et al.29. The amino acid residues that form a space for the UQI and UQII sites at the si-face of FAD are shown in transparent yellow meshes. (C,D) The overlap projection of ubiquinone in UQI and UQII sites with stigmatellin at STG-1a site (C) and stigmatellin at STG-1b, AC0-12 and myxothiazol (D). (E) The predicted UQI site in ubiquinone reduction by Ndi1. The structures determined for the Ndi1-stigmatellin, Ndi1-ubiquinone (PDB code: 4G74)29 and Ndi1-NAD+ complexes (PDB code: 4GAP)28 are superposed. The ubiquinone molecule (magenta) in the UQI site and NAD+ molecule (yellow) are located on the si-face and re-face of the isoalloxazine ring of FAD (white), respectively. The head group of stigmatellin in both positions of STG-1a (transparent aqua) and STG-1b (transparent green), as well as the alkenyl chain of stigmatellin in the position of STG-1a overlaps with the UQI site.