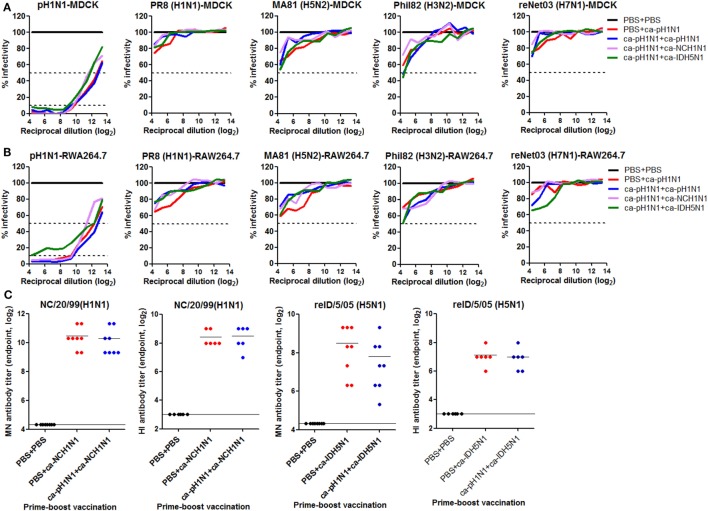
Figure 7.
Safety issues related to heterogeneity. (A,B) Vaccine-induced sera antibodies do not cause vaccination-associated enhanced respiratory disease (VAERD) by heterologous influenza viruses. Twofold serial dilutions of sera obtained from vaccinated mice (N = 5) were incubated with 100 tissue cell infectious dose 50 (TCID50) of each virus and the mixtures were absorbed into Madin-Darby canine kidney (MDCK) (A) or RAW264.7 (B) cells in a 96-well plate. Twenty-four hours later, the viral infectivity of each well was measured by NP-based ELISA protocol. Percent infectivity was calculated based on OD490 value compared to that of sera from the non-vaccinated mice. Data are the mean of each cohort. (C) Prime vaccination does not interfere with antibody generation by boost vaccination. Mice (N = 6 to 8) were vaccinated with PBS + ca-NCH1N1 or PBS + ca-IDH5N1, and sera microneutralization (MN) or hemagglutinin inhibition (HI) antibodies against homologous A/NC/20/1999 or reA/ID/5/2005 virus were estimated and compared to antibody titers generated by vaccination with ca-pH1N1 + ca-NCH1N1 or ca-pH1N1 + ca-IDH5N1.