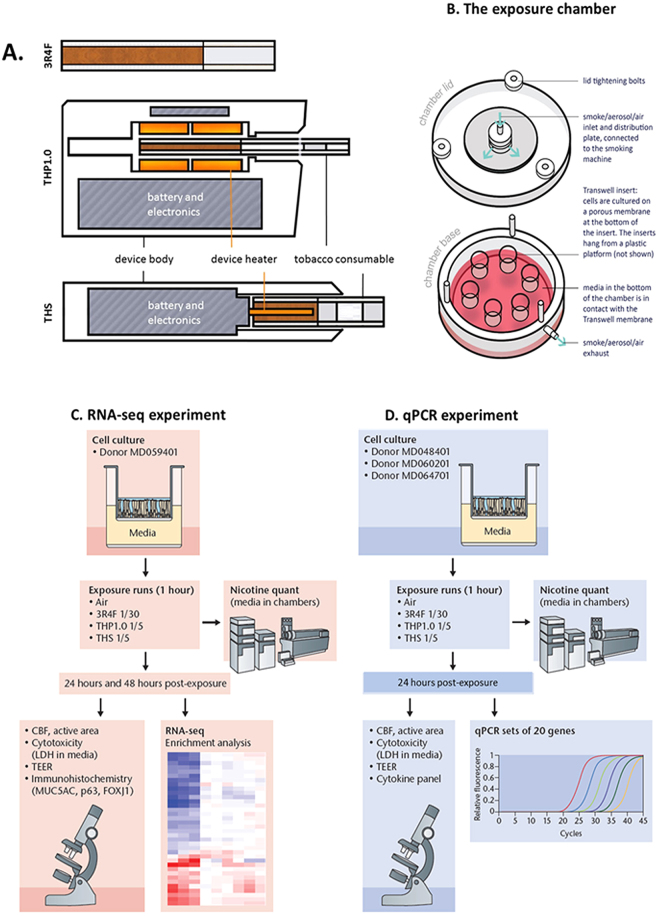
Figure 1.
Test product schematics and experimental design summary. (A) Cross section schematic representation of the 3 test products used in this study: 3R4F reference combustible cigarette; THP1.0 having a slim size tobacco consumable inserted in the heating device with the peripheral heating components and THS having a king size tobacco consumable with a centrally positioned heating blade. (B) Aerosol exposure chambers with the cell inserts position (Figure adapted from Haswell et al.13). (C) RNA-seq experimental design. MucilAirTM (donor MD059401) reconstituted airway tissues were exposed to aerosols from 3R4F, THP1.0, THS and to air at the indicated dilutions (Figure adapted from Haswell et al.13). The media in the exposure chambers were collected for nicotine quantification at the end of the exposure. Following 24 and 48 hrs post-exposure, MucilAirTM tissues were assessed for functional endpoints and RNA isolated for RNA-seq. A minimum of 3 independent repeats were conducted with 3 cell inserts per treatment. (D) Validation of results in multiple MucilAirTM donors by qPCR (Figure adapted from Haswell et al.13). Three different donors (MD048401, MD060201, MD064701) were exposed to aerosols of 3R4F, THP1.0, THS at the shown dilutions, and to air. Three technical replicates were used per donor and treatment. Nicotine was quantified as previously described. Functional endpoints were assessed in MucilAirTM and supernatants collected for cytokine quantification and total RNA extracted for qPCR at 24 hrs post-exposure.