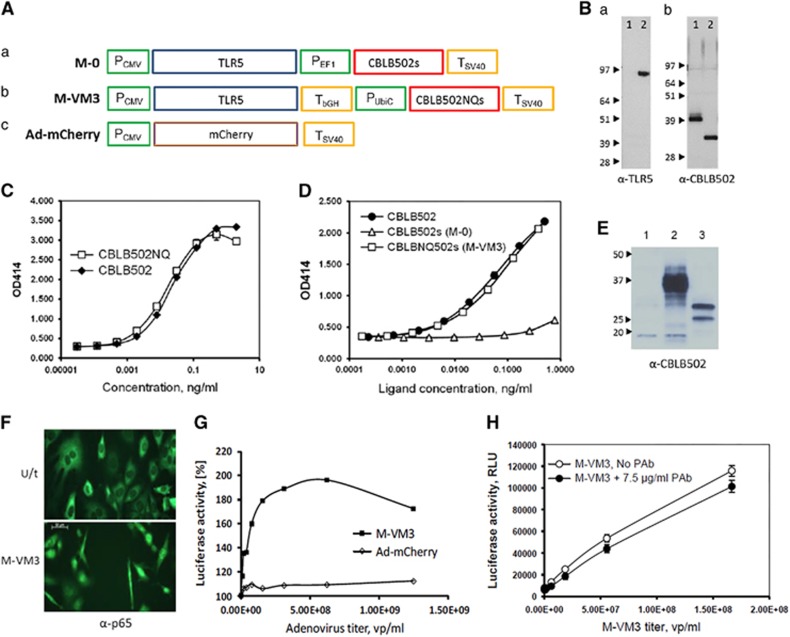
Figure 1.
Adenoviral constructs and their characterization in vitro. (A) Schematic representation of expression cassettes in (a) Mobilan M-0, (b) Mobilan M-VM3, and (c) Ad-mCherry. P, promoter, T, transcription terminator. (B) Western blot analysis of Mobilan-directed protein expression in MOSEC cells. (a) Detection with anti-TLR5 antibody; lysates from uninfected (lane 1) and M-0-infected MOSEC cells (lane 2). (b) Detection with rabbit anti-CBLB502 pAb; lysates from M-0-infected MOSEC cells left untreated (lane 1) or treated with a mixture of deglycosylation enzymes (lane 2). (C) Activity comparison of E. coli-produced CBLB502 and CBLB502NQs in HEK293-NF-κB-lacZ reporter cells. Cells were incubated with purified proteins for 24 h; β-galactosidase activity (OD414) was measured in cell lysates using ONPG substrate. (D) Activity comparison of CBLB502s and CBLB502NQs produced in MOSEC cells. MOSEC cells were infected with M-0 or M-VM3, medium was collected after 48 h and the concentrations of CBLB502s and CBLB502NQs were measured by ELISA after heat-inactivation of residual adenovirus. The indicated amounts of MOSEC-produced proteins or E. coli-produced CBLB502 standard were applied to HEK293-NF-κB-lacZ cells and β-galactosidase was measured as above. (E) Western blot analysis of MOSEC cells infected with Ad-mCherry (lane 1), M-0 (lane 2) or M-VM3 (lane 3) using rabbit anti-CBLB502 pAb. The protein with slower mobility in lane 3 is the expected size of unglycosylated CBLB502NQs (31.5 kDa); the faster mobility protein is presumably a partially degraded form of CBLB502NQs. (F) Infection with M-VM3 (MOI=3 × 104) induces NF-κB p65 nuclear translocation in TLR5-negative MOSEC cells 24 h after infection compared with control uninfected MOSEC cells. Antibodies against NF-κB p65 were used for immunostaining. (G) Induction of NF-κB-dependent luciferase expression in TRAMP-C2 cells infected by M-VM3. TRAMP-C2 cells carrying an NF-κB-dependent luciferase reporter construct were infected with M-VM3 or Ad-mCherry at the indicated MOIs. Luciferase activity was measured in lysates prepared 48 h post-infection and is shown as a percentage of that in uninfected cells (set at 100%). (H) Effect of neutralizing anti-CBLB502 antibodies on TLR5 signaling in M-VM3-infected MOSEC-NF-κB-luciferase reporter cells. Cells were infected with M-VM3 in the presence or absence of an excess of neutralizing rabbit anti-CBLB502 pAb. Luciferase activity was measured after 80-h incubation. Graphs show mean±s.d. of triplicate measurements.