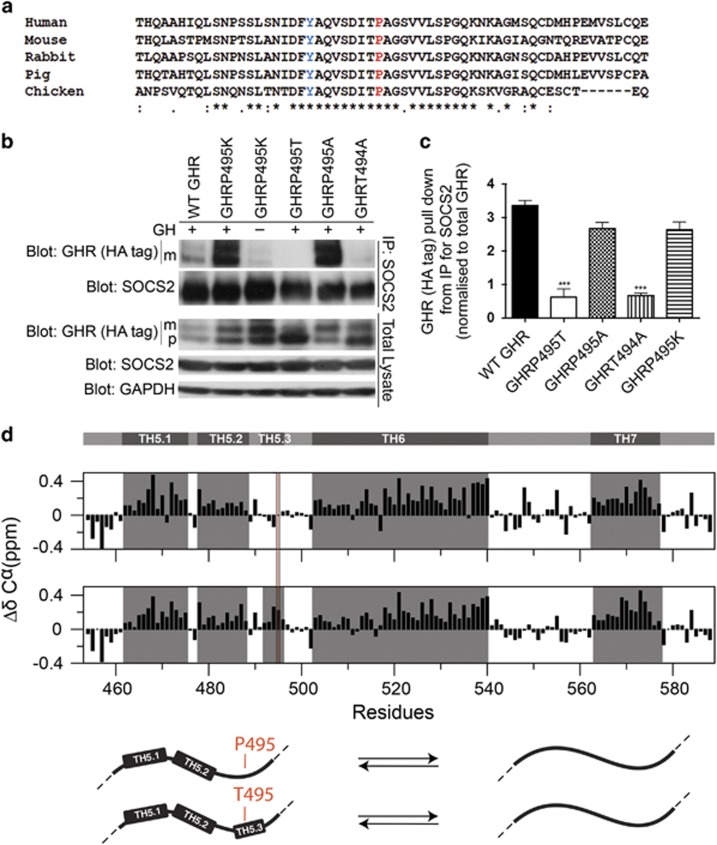
Figure 5.
GHRP495T generates structural changes in the receptor intracellular domain. (a) Clustal Omega multiple sequence alignment of the GHR polypeptide in close proximity to Pro 495 (red). The Tyr residue, a known active STAT5 binding site is coloured blue. Symbols below indicates (*) identical residues, (:) conservation of strongly similar properties and (.) conservation of weakly similar properties. (b) Importance of residues surrounding Pro495 to SOCS2 binding. HEK293 cells stably transduced with WT GHR, GHRP495T, GHRP495K, GHRP495A and GHRT494A were subjected to co-IP analysis as described in Materials and methods. Protein complexes from the immunoprecipitates were immunoblotted for GHR (anti-HA-tag) (mature (m) receptor and precursor (p) receptor) and SOCS2. As control, a small volume of total cell lysates used for co-IP was probed to indicate endogenous levels of GHR, SOCS2, and GAPDH (loading control). (c) Graph represents the signal intensity of total GHR (HA-tag) pull down relative to SOCS2 input, corrected for endogenous GHR expression. Data presented as mean±s.e.m. analysed by one-way ANOVA (***P<0.001) and representative of three independent experiments confirmed in three lines generated by independent transductions. (d) GHRP495T changes the structural ensemble in the GHR intracellular domain by nuclear magnetic resonance spectroscopy analysis. Secondary Cα-chemical shift (SCS) of GHR ICD455-588WT (top panel). Consecutive positive SCS-values indicate transiently folded helices (TH) marked in grey boxes. The original TH5, as previously reported34 was re-evaluated as two interrupted transient helices as TH5.1 (E462-L475), TH5.2 (P478-S488), and are shown together with TH6 and TH7 (top panel). SCS of GHR ICD455-588P495T (middle panel). P495T induces helicity around the mutation site and before in the C-terminal of TH5.2. Numbering includes the signal peptide. Model illustrating the change in structural ensemble around T495 (bottom panel) and the inter-conversion equilibrium between disordered and helical structures, where the helicity is increased in the GHR ICD455-588P495T.