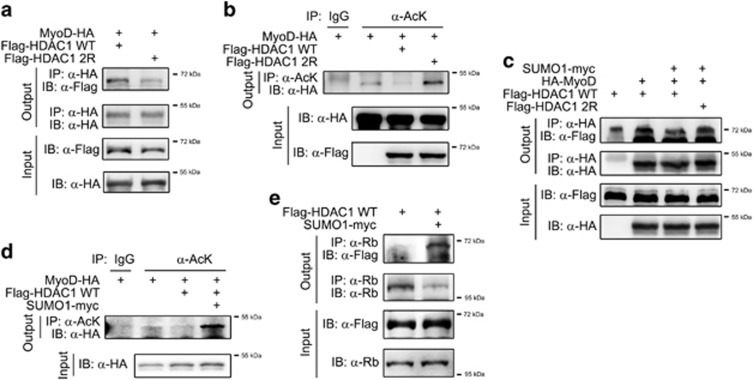
Figure 5.
Histone deacetylase 1 (HDAC1) sumoylation is required for its deacetylase activity of MyoD during myoblast differentiation. (a) The association between HDAC1 and MyoD is slightly reduced in HDAC1 2R. Immunoprecipitation was performed with either HDAC1 WT or HDAC2 2R in 293T cells. The association between HDAC1 2R and MyoD was reduced (first panel). Notably, the input amount of HDAC1 2R is also less than that of HDAC1 WT (third panel). (b) MyoD deacetylation is dependent on HDAC1 sumoylation. Acetylation of MyoD was measured by immunoprecipitation-based analysis by utilizing anti-acetyl lysine antibody (α-AcK) in 293T cells. Transfection of HDAC1 WT decreased the acetylation of MyoD (third lane), suggesting the intact deacetylase activity of HDAC1. HDAC1 2R failed to deacetylate MyoD (fourth lane). (c) Forced sumoylation of HDAC1 caused its dissociation from MyoD. We transfected Flag-HDAC1 WT, MyoD-HA and SUMO1-Flag into 293T cells as indicated and immunoprecipitation assay was performed. Note that forced sumoylation reduced the interaction between MyoD and HDAC1 (second vs third lanes in the uppermost panel). (d) Forced sumoylation enhanced acetylation of MyoD. Transfection of HDAC1 WT induced deacetylation of MyoD (second vs third lanes), whereas co-transfection of small ubiquitin-like modifier 1 (SUMO1) restored the acetylation of MyoD in 293T cells. Acetylation of MyoD was measured by immunoprecipitation. (e) Forced sumoylation enhanced the binding of HDAC1 to Rb in C2C12 cells.