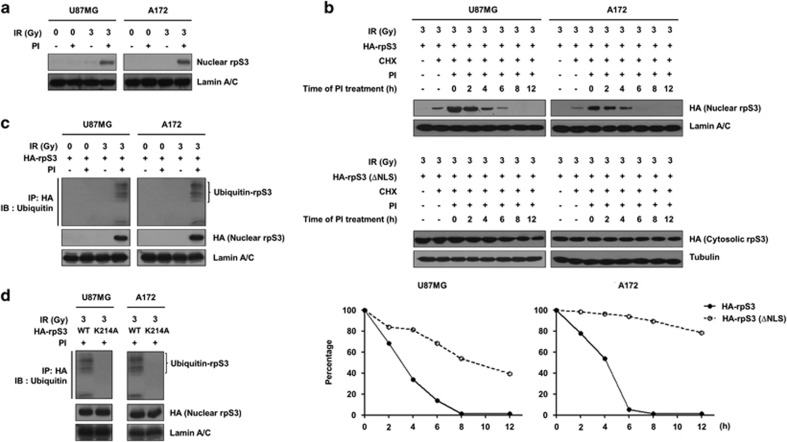
Figure 2.
Poly-ubiquitination is required for degradation of rpS3 in irradiated GBM cells. (a) The effects of proteasome inhibition on the protein stability of nuclear rpS3 were measured by western blotting (proteasome inhibitors, PI; a mix of 5 mmol l−1 MG115 and 5 mmol l−1 MG262, 24 h incubation). (b) The half-life of rpS3 WT or mut (ΔNLS) in response to radiation with cycloheximide (CHX; 50 mmol l−1) in the presence of PI was estimated by western blotting. The cells were treated with PI at different time points after irradiation (0–12 h). The protein levels of HA-rpS3 WT or ΔNLS at each time point measured in b were analyzed by densitometric analysis. The expression level of HA-rpS3 WT or ΔNLS at time point 0 (lane 3) was set at 100%. (c) The accumulation of poly-ubiquitinated nuclear rpS3 in response to radiation was measured. U87MG and A172 cells expressing HA-rpS3 were treated with or without PI. HA-rpS3 protein was immunoprecipitated with an anti-HA antibody, and western blotting was performed with an anti-ubiquitin antibody. (d) The effects of the Lys214 residue on the poly-ubiquitination of rpS3 were measured. U87MG and A172 cells expressing rpS3 WT or mut (K214A) were treated with PI. HA-rpS3 protein was immunoprecipitated with an anti-HA antibody, and western blotting was performed with an anti-ubiquitin antibody.