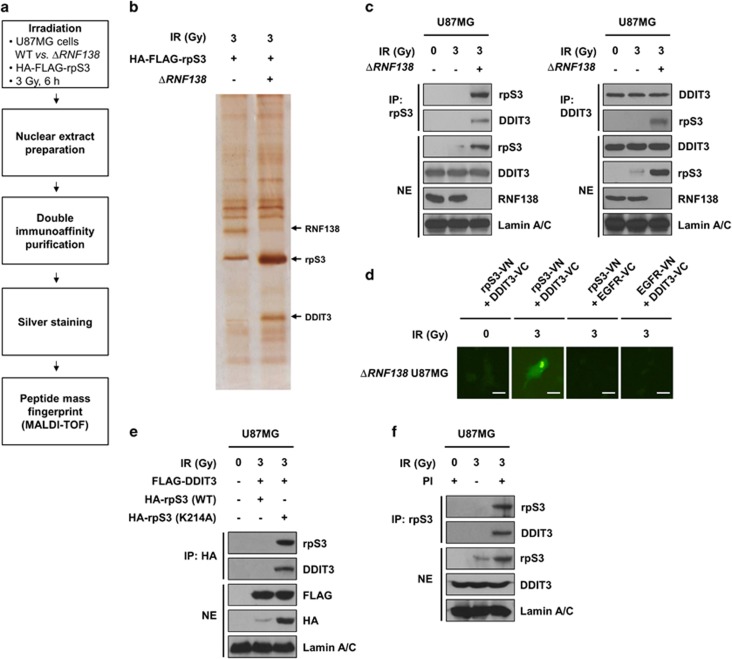
Figure 4.
RpS3 ubiquitination negatively affects radiation-induced DDIT3 signaling in GBM cells. (a) Schematic presentation of the strategy used to identify the rpS3 nuclear interactome in U87MG cells. To generate ΔRNF138 U87MG cells, the CRISPR/Cas9 system was employed. (b) After irradiation, double-immuno-purified rpS3 complexes in normal U87MG or ΔRNF138 U87MG cells were separated by PAGE and visualized by silver staining. The silver-stained gel was analyzed by mass spectrometry (PMF). It was shown that three bands were particularly increased by irradiation. The bands indicated by arrowheads correspond to RNF138, rpS3, and DDIT3, respectively. (c) RpS3–DDIT3 interaction in normal U87MG and ΔRNF138 U87MG cells in response to irradiation was measured by reciprocal IP assay. (d) A BiFC assay was performed to evaluate the interaction of rpS3-DDIT3 in live cells. The ΔRNF138 U87MG cells were transiently transfected with pBiFC-rpS3-VN, pBiFC-DDIT3-VC, pBiFC-EGFR-VN and/or pBiFC-EGFR-VC. Fluorescence indicative of rpS3-DDIT3 binding was measured in irradiated ΔRNF138 U87MG cells. Scale bars, 25 μm. (e) The effects of rpS3 WT or mut (K214A) in response to irradiation on the interaction with DDIT3 were measured. (f) The effects of rpS3 degradation on the interaction with DDIT3 were determined.