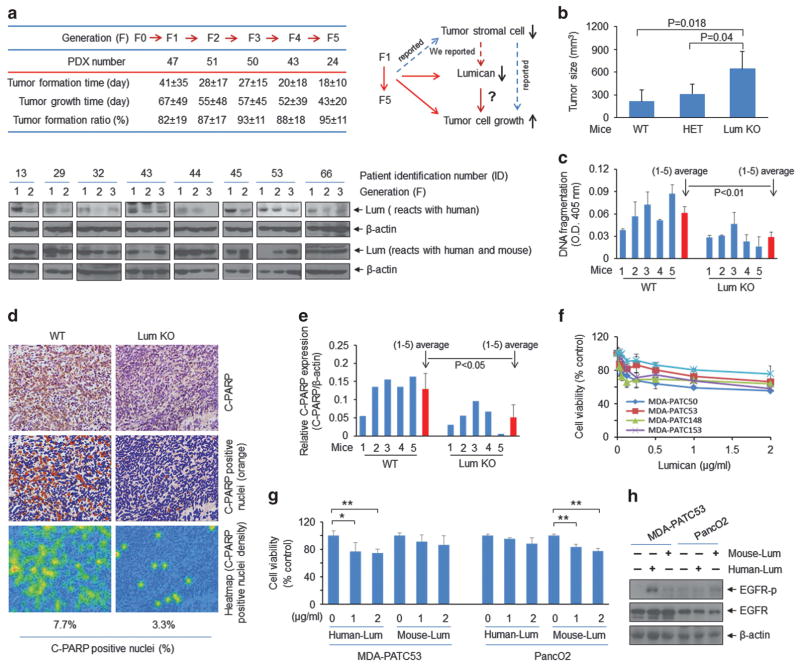
Figure 1.
Stromal lumican inhibits tumour growth. (a) Upper-left: patient tumour tissues (F0) were implanted into immunodeficient mice to obtain direct xenograft tumours (PDX: F1, F2, F3, F4, and F5). PDX number: statistical number of PDX from different patients. Tumour formation time: implantation to the tumour reached 0.25 cm in diameter; tumour growth time: tumour grows from 0.25 cm to 1.5 cm. Tumour formation ratio (%): tumour formation number/implanted number. Female NOD/SCID mice (4–8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). All animal studies were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee (IRB: Lab00-396. ACUF: 00001089-RN00). A blind analysis was performed in animal data statistical analysis. The protocols of engraftment and expansion of PDX tumours were performed as previously described.23,24 Briefly, excised patient tumour tissue (F0) was mechanically minced into fragments (about 2 mm), and five tumour fragments were individually placed in a formed tissue pocket. Once tumours reached 1.5 cm in greatest diameter, mice were killed, and the tumours were dissected (F1). F1 tumour tissue fragments were placed into NOD/SCID mice to generate the F2 to F5 generations. Lower: western blot analysis showed the lumican expression in tumour tissues from different PDX generations. Antibodies used were lumican (ab70191, Abcam, Cambridge, MA, USA) and β-actin (sc-69879, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Upper-right: schematic drawing depicts the conclusions from the chart (left), our previous findings, reported results and our hypothesis for the current study. (b) Tumour size was measured in orthotopic syngeneic mouse PDAC models. WT: wild-type mice; HET: heterozygous mice; Lum KO: lumican knockout mice. Freshly cultured PancO2 cells (purchased from ATCC) were re-suspended in sterile 1X PBS at 1 ×106 cells per 100 μl, and this volume of the suspension was directly injected into the pancreas of wild-type (WT) and lumican knockout (KO) C57BL/6 mice aged approximately 10 weeks, and were randomly placed into different groups ( six mice per condition). Lumican WT and KO mice were purchased from Jackson Laboratory. All mice were killed 5 weeks after PancO2 cells injection. (c) Tumour tissue lysates were subjected to a cell death detection ELISA kit (11774425001, Roche, Mannheim, Germany). (d) Immunohistochemistry staining of tumour tissues from lumican WT and KO mice with anti-cleaved PARP antibody (ab32064, Abcam). The C-PARP labelled pancreatic tumour tissues were scanned using Olympus BX51. Within DAB-stained slides, six random fields in tumours were scanned at× 10. The obtained images were analysed using the ‘Nucleus detection, Nucleus Morphology and Filter, and Nucleus classification’ algorithm in Tissue Studio 2.5 (Definiens, Cambridge, MA, USA). The C-PARP positive nuclei were quantified. (e) Tumour tissue lysates were subjected to western blot analysis with cleaved PARP (C-PARP) antibody (#9542, Cell Signaling Technology, Beverly, MA, USA). Quantification of C-PARP protein level was normalized to β-actin. The Student’s t-test was used (in (c) and (e)) to detect the significance of differences between groups. The data are presented as means ± s.d. All experiments were repeated at least once with reproducible findings. (f) Cell viability was measured by methylthiazolyldiphenyl-tetrazolium bromide (MTT) (M2128, Sigma-Aldrich, St Louis, MO, USA) assay after lumican (recombinant human lumican protein CF, R&D Systems, 2846-LU-050, Minneapolis, MN, USA) treatment for 3 days. Primary PDAC cell lines MDA-PATC50, MDA-PATC53, MDA-PATC148 and MDA-PATC153 were isolated from PDX tumours in our laboratory.10 All cell lines were verified by DNA fingerprinting at the Characterized Cell Line Core Facility of the MD Anderson Cancer Center. The OD value of each treatment group was expressed as a percentage of the OD value of the untreated control cells. (g) Human MDA-PATC53 cells and mouse PancO2 cells were treated with human recombinant lumican protein and mouse recombinant lumican protein (recombinant mouse lumican protein CF, R&D Systems, 2745-LU-050) with indicated doses for 3 days, respectively. Cell viability was measured by MTT assay. *P<0.05 and **P<0.01. (h) MDA-PATC53 and PancO2 cells were treated with 2 μg/ml human lumican or mouse lumican for 10 min, respectively. Cell lysates were subjected to western blot analysis with anti-phospho-EGF receptor (#2234, Cell Signaling Technology), anti-EGFR (sc-373746, Santa Cruz Biotechnology) and anti-β-actin.