Figure 1.
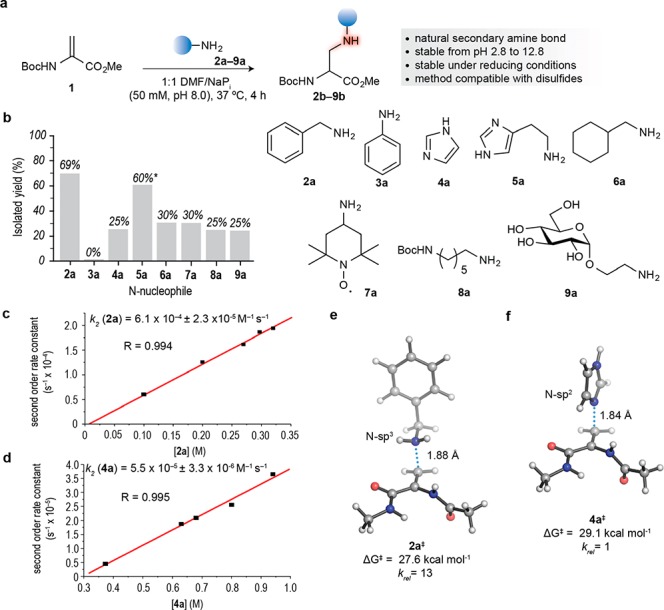
Reaction between a protected Dha amino acid derivative and amine nucleophiles. (a) Reaction of Boc-Dha methyl ester 1 with N-nucleophiles. (b) Graphical representation of the isolated yields of the reaction of 1 and N-nucleophiles 2a–9a. General conditions for amine addition to Dha: Boc-Dha methyl ester 1 (1 equiv) and N-nucleophile 2a–9a (1.5 equiv) in a 1:1 mixture of DMF/sodium phosphate buffer (50 mM, pH 8.0) at 37 °C for 4 h. All yields were calculated after SiO2 flash column chromatography purification with the exception of the addition of 5a to 1 for which conversion using the crude mixture is indicated* (1.7:1 N-sp3/N-sp2 ratio). (c,d) Experimental determination of the second order rate constant for the addition of benzylamine 2a and imidazole 4a to Boc-Dha methyl ester 1, respectively. (e,f) Transition structures and associated activation free energies (ΔG‡) at 25 °C and relative reaction rates (krel) calculated with PCM(water)/M06-2X/6-311+g(2d,p) for the aza-Michael ligation of model dehydro amino acid Ac-Dha-NHMe with benzylamine 2a and imidazole 4a, respectively. NaPi, sodium phosphate buffer; DMF, dimethylformamide; Boc, tert-butyloxycarbonyl.
