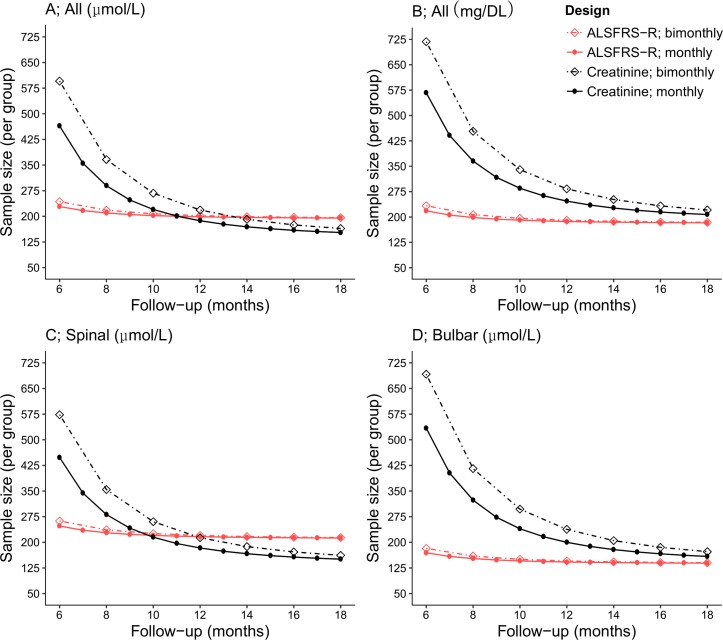
Figure 3.
Sample size and power calculations for plasma creatinine and the ALSFRS-R. Sample size calculations were performed for the ALSFRS-R and plasma creatinine, with varying total follow-up durations (ranging 6–18 months) and with either bimonthly or monthly return visits. All calculations were based on an expected standardised 30% reduction in slope during follow-up, with a two-sided alpha of 5% and power of 90%. For short trials (up to 10 months), the smaller between-patient variability at baseline and relatively faster rate of decline of the ALSFRS-R (see online supplementary eTable 1) resulted in smaller sample sizes. For longer trials (>10 months), sample size calculations for plasma creatinine, when determined in micromoles per litre, resulted in smaller sample sizes due to the lower variability between patients in rate of decline (A). When plasma creatinine levels were determined in micrograms per decilitre, this advantage was lost (B). The utility of plasma creatinine seems higher in spinal-onset patients (C) as compared with bulbar-onset patients (D). ALSFRS-R, amyotrophic lateral sclerosis functional rating scale–revised.