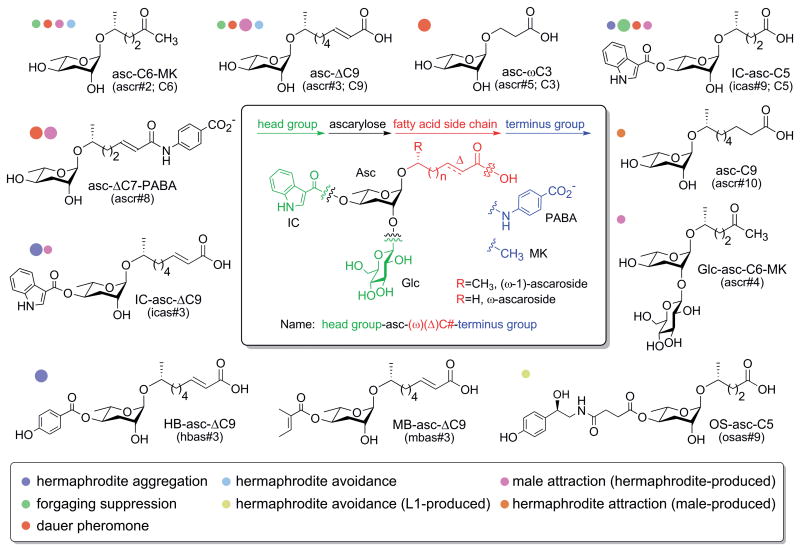
Figure 1.
Chemical structures of some key ascaroside pheromones. Colored dots next to the ascaroside structures indicate whether a particular ascaroside has been shown to have a particular activity. The size of the dot gives an indication of the relative strength of that activity compared to other ascarosides with that activity. Many of the ascarosides shown have not been tested for all activities listed so this figure does not give a complete activity profile many of the ascarosides. All of the ascarosides share a modular structure that is diagrammed in the central box. The ascarosides can be named based on this modular structure, and this structure-based name is listed below each ascaroside. The ascarosides can also be named using a name based primarily on the order of discovery (ascr#, icas#, mbas#, hbas#, osas#) or a name based on an earlier nomenclature (C6, C9, C3, or C5, indicating only the number of carbons in the side chain). For the structure-based name, the simplest ascarosides have an ascarylose sugar attached to a saturated fatty acid side chain of a particular length (C#) at its penultimate (ω-1) carbon. Deviations from that simple structure are indicated as follows: head group-asc-(ω)(Δ)C#-terminus group. ω indicates attachment at the terminal (ω), instead of the penultimate (ω-1) carbon. Δ indicates a double bond at the α-β position. Head groups include indole-3-carbonyl (IC), 4-hydroxybenzoyl (HB), 2-(E)-methyl-2-butenoyl (MB), and octopamine succinyl (OS) at the 4′-position and glucosyl (Glc) at the 2′-position. Terminus groups include a methyl ketone (MK) and para-aminobenzoic acid (PABA) instead of a carboxylic acid. The figure in the central box is adapted from ref. 26.