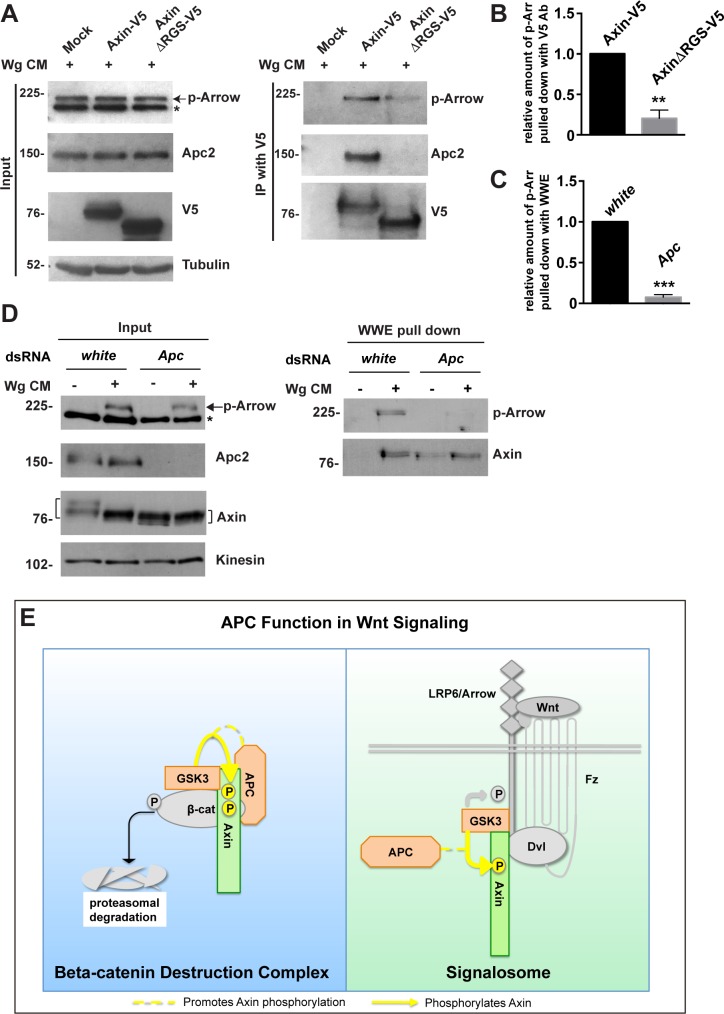
Fig 7. Apc promotes the association between Axin and phospho-LRP upon Wingless stimulation.
(A) S2R+ cells were transfected with the indicated plasmids, and treated with Wg CM 48 hours later for one hour. Lysates were subjected to immunoprecipitation with V5 antibody and analyzed by immunoblot. Deletion of the Apc binding domain of Axin (AxinΔRGS-V5) reduced the interaction between Axin and phosphorylated LRP6/Arrow after Wingless stimulation, as revealed by immunoblot with phospho-LRP6 antibody. Tubulin was used as a loading control. (B) Quantification of relative levels of phospho-Arrow pulled down with Axin-V5 from experiment shown in (A). Error bars represent s.e.m. of three independent experiments. P = 0.0054. (C) Quantification of relative levels of phospho-Arrow pulled down with WWE from experiment shown in (D). Error bars represent s.e.m. of three independent experiments. P = 0.0004. (D) S2R+ cells were treated with the indicated dsRNAs, followed by treatment with control medium or Wg CM for one hour. Lysates were subjected to GST-WWE pull down and analyzed by immunoblot. Treatment with Wg CM markedly increases the amount of ADP-ribosylated Axin pulled down with GST-WWE. Apc knockdown abolishes the Wg-dependent increase in ADP-ribosylated Axin pulled down with GST-WWE. Upon treatment with Wg CM, phospho-Arrow is also pulled down with GST-WWE, but is significantly reduced by Apc knockdown. Kinesin was used as a loading control. (E) Working model for Apc function in Wnt signaling. Apc promotes GSK3-catalyzed Axin phosphorylation in both Wnt-off and Wnt-on states, the rapid transition in Axin following Wnt stimulation, and Axin’s subsequent association with the Wnt co-receptor LRP6/Arrow, one of the earliest steps in pathway activation. We propose that this requirement for APC in Axin regulation through phosphorylation both prevents signaling in the Wnt-off state and promotes signaling immediately following Wnt stimulation.