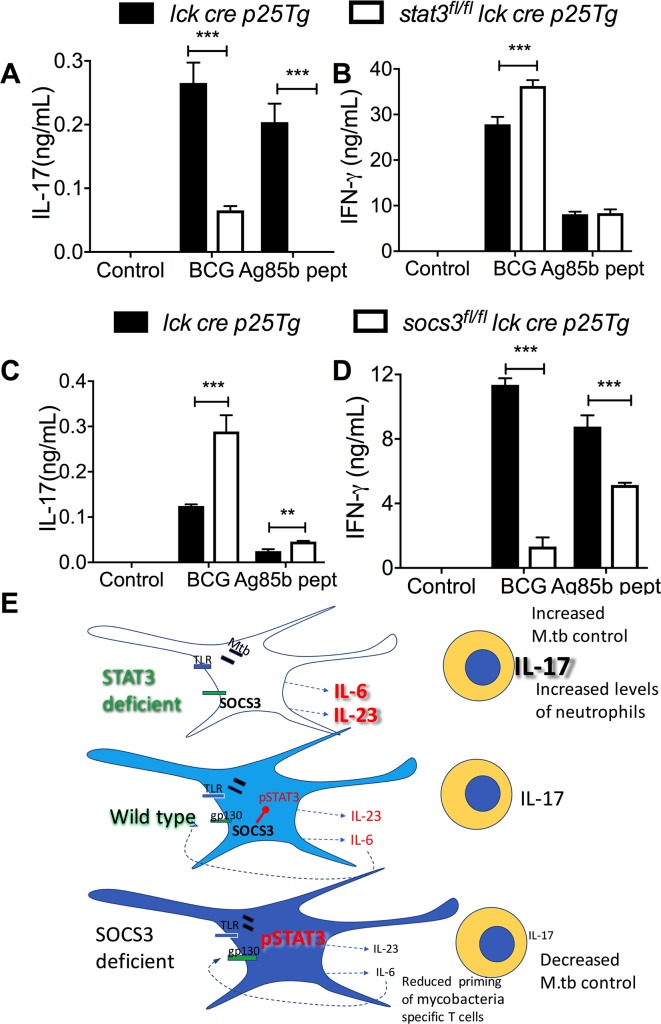
Fig 7. SOCS3 and STAT3 in antigen-specific T cells are important regulators of IL-17 and IFN-γ secretion.
Stat3fl/fl lck cre p25-tg T cells (A, B), socs3fl/fl lck cre p25-tg T cells (C, D) or control p25-tg T cells were incubated for 3 days with either BCG, peptide 25 and LPS-stimulated or untreated BMDCs. The mean concentration of IL-17 (A, C) and IFN-γ (B, D) ± SEM in supernatants from triplicate cultures is shown (**p<0.01 and ***p<0.001 Student’s t test). Graphical summary (E): Mice deficient in STAT3 in myeloid cells show increased resistance while socs3fl/fl lysm cre mice displaying augmented STAT3 activation had impaired resistance against infection with M. tuberculosis. The differential control of infection in excess or deficiency of STAT3 is not due to the intrinsic regulation of bacterial control by macrophages but rather to differences in the ability of DCs to regulate the differentiation of specific T-cells. Stat3fl/fl lysm cre APCs release higher levels of IL-6 and IL-23 after stimulation with mycobacterial or other TLR agonists, while secretion of these cytokines is reduced in socs3fl/fl lysm cre APCs. Stat3fl/fl lysm cre APCs show improved ability to trigger IL-17 release by mycobacteria-specific T cells while the opposite is observed when using socs3fl/fl lysm cre APCs. The IL-17 secretion by T cells is also controlled via gp130R signalling, indicating an autocrine or paracrine loop by IL-6 family cytokines. The increased resistance to M. tuberculosis infection of stat3fl/fl lysm cre mice was IL-17 dependent. Socs3fl/fl lysm cre (but not stat3fl/fl lysm cre) DCs improved capacity of mycobacteria-specific T cells priming in vivo.