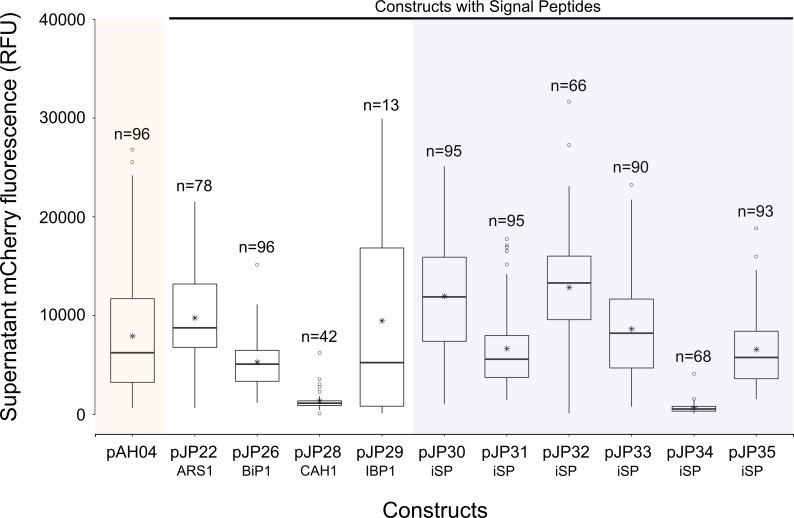
Fig 3. Comparison of mCherry fluorescence in the supernatant of different constructs.
mCherry fluorescence was measured in supernatant. mCherry fluorescence was measured from 96 individual colonies, grown in a deep-well plate in TAP media for 7 d under constant illumination and agitation. Data presented in the boxplot were collected from colonies where the total fluorescence signal was higher than the auto-fluorescence of the parental wild-type cc1690, above three standard deviations. pAH04 –construct without SP; pJP22 –construct with arylsulfatase 1 SP; pJP26 –construct with binding protein 1 SP; pJP28 –construct with carbonic anhydrase 1 SP; pJP29 –construct with ice-binding protein 1 SP; pJP30-35 –construct with in silico identified SP (iSP). n–number of positive results obtained for each construct. * represents the average result. ○ represents the outliers. No normalization was conducted for mCherry fluorescence.To confirm this hypothesis, a positive colony of each construct with the highest fluorescence was cultivated in 50 mL of TAP media for 7 d, and mCherry fluorescence was determined in both the total cultures and in the supernatant (S2 Fig). The supernatant percentage of mCherry fluorescence in the pAH04 strain cultivated in the flask was lower relative to that of the pAH0A strain cultivated on the plate (from 42% on the plate to ~8.5% in the flask), which was consistent with the culture condition hypothesis. Although the test in the flask presented a lower noise, it lacks the throughput to test several colonies, an important feature when comparing different construct designs. Since transformation is based mainly on a random insertion by non-homologous end joining (NHEJ) [39], colonies presented a wide range of expression efficiency, from a relative standard deviation of 42.7% to 102.7% (S1 Table). Therefore, we chose the 96 well plate assay to compare constructs efficiency since it could prevent sampling bias.