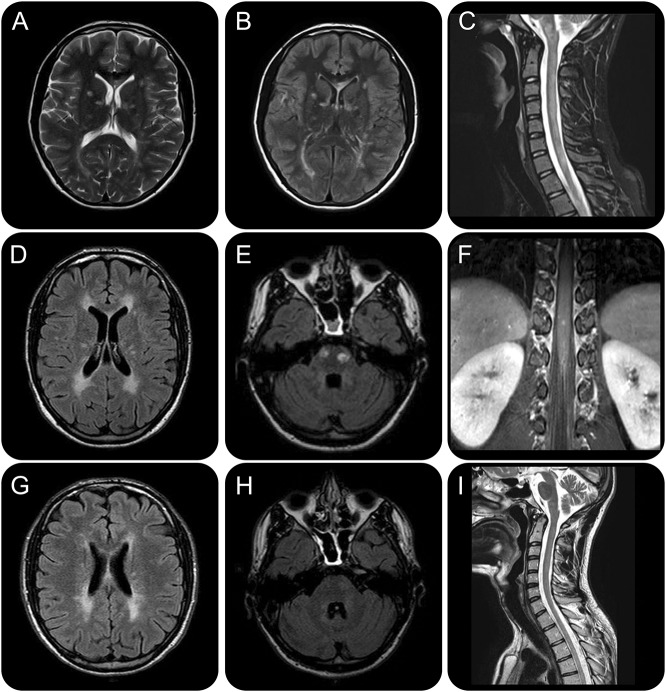
Figure 1. Brain and spine MRI findings at baseline, after natalizumab discontinuation, and at 12-month follow-up after rituximab administration.
Brain and spine MRI findings at baseline (A–C), after natalizumab discontinuation (D–F), and at 12-month follow-up after rituximab administration (G–I), and EDSS timeline. Baseline T2-weighted (A) and fluid-attenuated inversion recovery (FLAIR) (B) axial brain sections highlight multiple hyperintense signal alterations mainly in subcortical and periventricular white matter and corpus callosum, associated with a T2-hyperintense cervical spinal cord lesion extending longitudinally from C2 to C7 (C) and other smaller dorsal lesions (D1 and D7–D8) and cauda equina roots hypertrophy. Diffuse signal alterations were also found in cerebellum and brainstem (not shown): these findings globally fulfilled the 2010 revised multiple sclerosis (MS) criteria for dissemination in space and time, although longitudinally extensive transverse myelitis and cauda equina involvement are highly atypical and made the diagnosis of MS unlikely. After an initial clinical and neuroradiologic improvement with natalizumab, the patient experienced a new severe relapse with prominent peripheral nervous system (PNS) deterioration and new brain (i.e., periventricular white matter, corpus callosum, internal capsule, pons) (D) and dorsal-lumbar spine lesions with contrast enhancement on MRI. Therapy was therefore discontinued. Brain axial FLAIR image highlights 2 ovoid hyperintense signal alterations in the pons (E); spine coronal T1-weighted image shows cauda nerve roots and conus medulla lesions with contrast enhancement (F). We decided to start rituximab as a third-line rescue treatment; clinical response on both CNS and PNS was rapid and persistent. MRI after 12 months of clinical stability evidenced a global reduction in volume and number of the demyelinating lesions, without new signal alterations. Brain axial FLAIR images highlight slight reduction of anterior periventricular white matter lesions (G) and marked reduction of the pons lesions (H). Spine sagittal T2-weighted image shows great improvement of the cervical lesion (I).