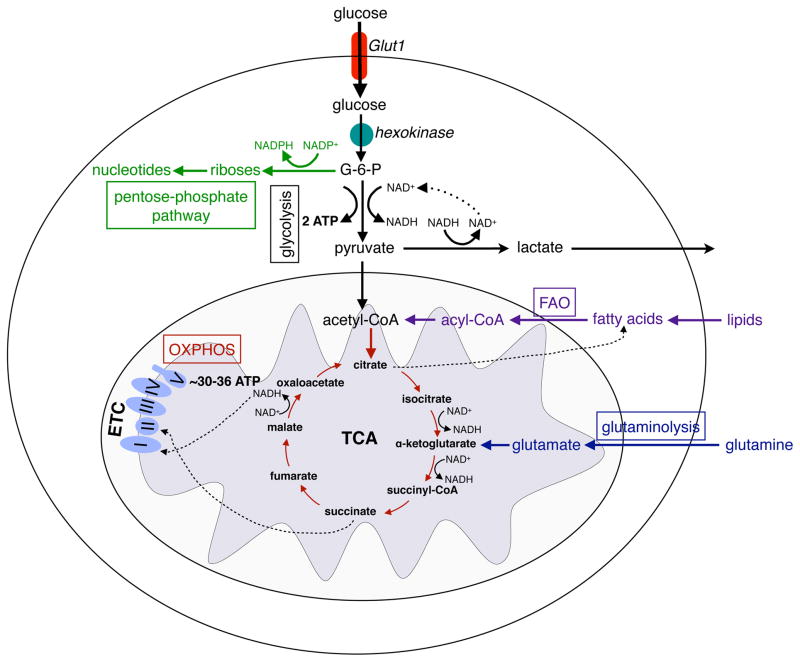
Figure 1. Major metabolic pathways of immune cells.
Glucose enters the cells through the glucose transporter, GLUT1 and is phosphorylated to glucose-6-phosphate (G-6-P) by hexokinases. During glycolysis, G-6-P is metabolized to pyruvate, reducing NAD+ to NADH, and generating 2 ATP. In hypoxia, pyruvate is oxidized to lactate, restoring NAD+ levels in the cell. In normoxia, pyruvate is metabolized to acetyl-CoA, which is oxidized in the TCA cycle to generate NADH. In the redox reactions of OXPHOS, electrons are sequentially transferred to generate a H+ gradient across the inner mitochondrial membrane, which drives the synthesis of ATP. In contrast to glycolysis, mitochondrial OXPHOS is a highly efficient form of generating ATP, yielding ~30–36 ATP per molecule of glucose. Immune cells utilize three additional pathways, the pentose phosphate shunt (PPP), glutaminolysis, and fatty acid oxidation, to meet their metabolic and functional demands. G-6-P is the entry point for PPP, which generate riboses for nucleotide synthesis. During this process, NADP+ is reduced to NADPH, forming the critical co-factor required for ROS production via the NADPH oxidase system in neutrophils and macrophages. During glutaminolysis, glutamine is metabolized to glutamate and subsequently toα -ketoglutarate, which then enters the TCA cycle. The fate of glutamine is dependent on the activation state of the immune cell; it can either be oxidized completely to generate ATP or used to replenish the metabolic intermediates of TCA cycles, which are diverted for macromolecule biosynthesis. The β-oxidation of fatty acids yields acetyl-CoA, which enter the TCA cycle and OXPHOS pathways to generate ATP.