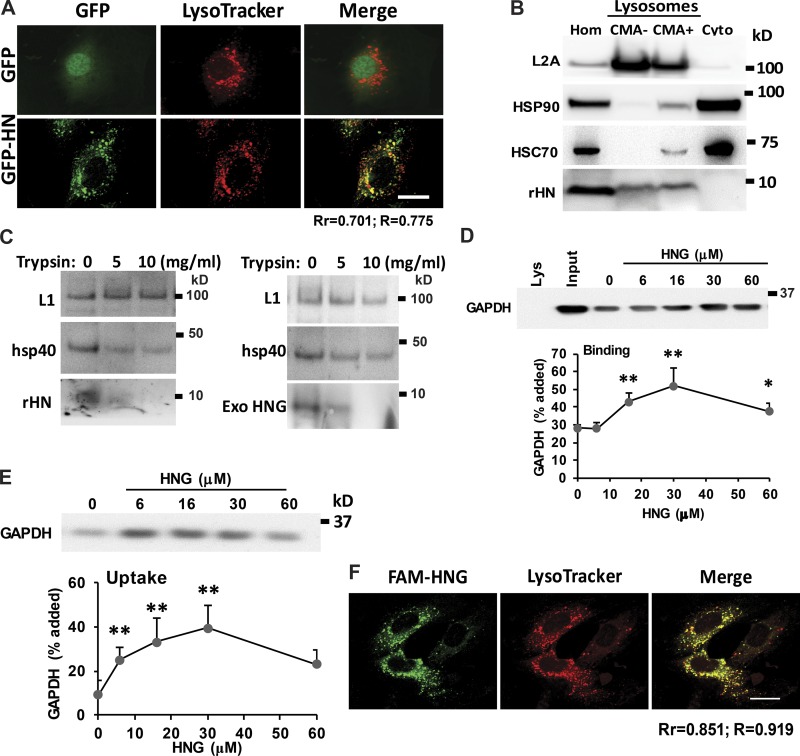
Figure 5.
HN localizes to lysosomal surface and increases CMA substrate uptake into the lysosomes. (A) Colocalization of HN and lysosomes in H9C2 cells. (B) Immunoblot for rHN, HSP90, and L2A in homogenate (Hom), cytosol (Cyto), and lysosomes with low (CMA−) and high (CMA+) CMA activity isolated from rat liver. (C) Immunoblot for the indicated proteins of rat liver lysosomes untreated (left) or preincubated with HNG (right) and incubated with the indicated concentrations of trypsin for 10 min at 25°C. Immunoblots for rHN (left) or HNG (right). LAMP-1 (L1) and hsp40 are shown as examples of luminal and surface-associated proteins, respectively. (D and E) Intact rat liver lysosomes were preincubated (E) or not (D) with protease inhibitors or were incubated with a fixed concentration of GAPDH in the presence of increasing concentrations of HNG. At the end of the incubation, lysosomes were recovered by centrifugation and subjected to immunoblotting for GAPDH. Representative immunoblots and quantifications of the binding (D) and uptake (E) calculated from the amount of GAPDH added to the incubation media bound to lysosomes without protease inhibitors (binding) or the difference in association between lysosomes preincubated or not with protease inhibitors (uptake). (F) Colocalization of exogenous added FAM-labeled HNG with lysosomes in H9C2 cells. Bars, 10 µm. Colocalization was analyzed in both Pearson’s correlation coefficient (Rr) and Manders’s overlap coefficient (R). Values are means and SEM of three to five different experiments. Differences with untreated control samples were significant for *, P < 0.05; **, P < 0.01.