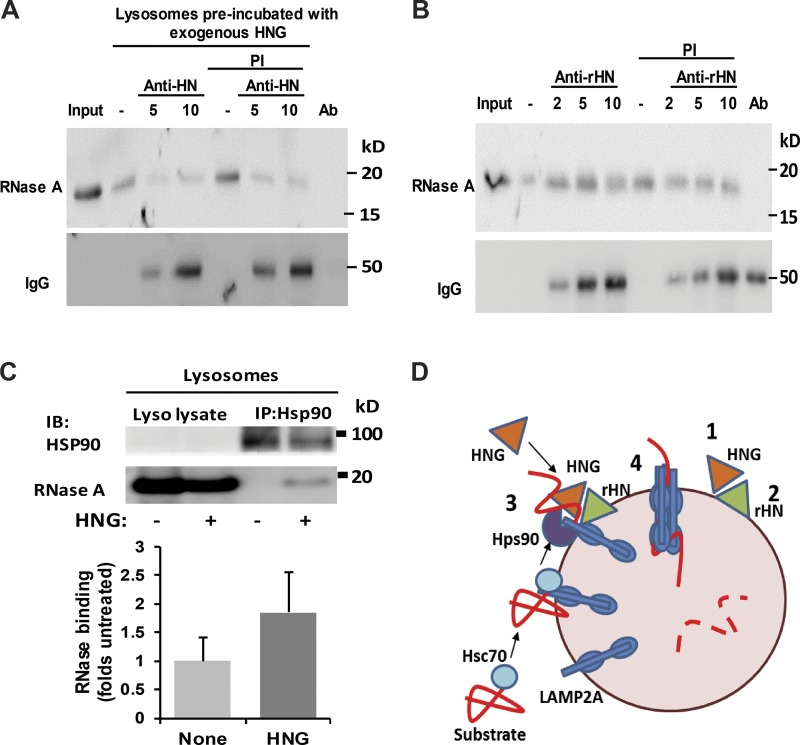
Figure 8.
Effect of lysosome-associated HN on CMA. (A) Rat liver lysosomes were preincubated with HNG to allow for its binding to the lysosomal membrane and, after centrifugation to eliminate unbound HNG, were incubated with increasing concentrations of the antibody against HN. After the treatment, lysosomes were incubated in the presence or absence of protease inhibitor (PI) with RNase A and subjected to immunoblot against RNase A. (B) Rat liver lysosomes were preincubated with increasing concentrations of the antibody against rHN and then with RNase A in the presence or absence of protease inhibitor. Samples were processed as in A. Secondary antibody was used to confirm binding of antibodies against HN (A) or rHN (B) to the lysosomal membrane. Ab: antibody only. (C) Rat liver lysosomes incubated with RNases A in presence (+) or absence (−) of HNG were subjected to immunoprecipitation (IP) for HSP90 and immunoblot (IB) for HSP90 and RNase A. Top: Representative immunoblot. Bottom: Quantification of the amount of RNase coimmunoprecipitated with HNG. Values are expressed as folds of untreated lysosomes. Error bars show SEM. (D) Proposed model of the stimulatory effect of HN on CMA: Cytosolic HNG enhances CMA by stabilizing the interaction of HSP90 with the CMA substrates while LAMP2A transitions into the multimeric complex carrying along the substrate proteins. A fraction of the endogenous HN (rHN in our study) associated to the lysosomal membrane is required to complete substrate translocation.