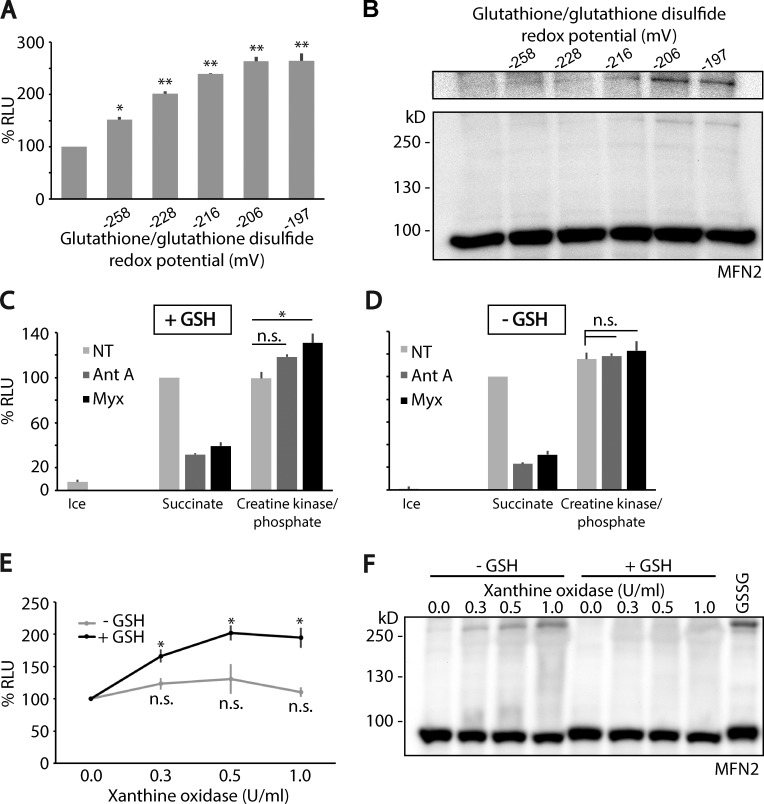
Figure 3.
Mfn2 is a sensor of mitochondria oxidative stress through GSSG, which activates mitochondrial fusion. (A) Mitochondria containing each half of the matrix-targeted split-luciferase probes were incubated within the cell-free fusion assay system with different ratios of GSH:GSSG, corresponding to increasing GSH redox potential. Mitochondrial fusion under these conditions was quantified by measuring luciferase activity. Luciferase counts were normalized to standard condition. Fusion is activated as the potential becomes oxidizing. Reported values are the mean of three biological replicates, each performed in duplicate. Error bars are means ± SEM. (B) Fusion samples from A were analyzed under denaturing, nonreducing SDS-PAGE followed by immunoblotting against Mfn2 (Abnova). This gel system denatures proteins yet preserves disulfide bonds. A gradual appearance of Mfn2 disulfide oligomers was observed corresponding to fusion activation. (C and D) Mitochondrial fusion assays were performed with succinate to drive respiration. This reaction for 30 min at 37°C was set as the basal 100%, against which the other reactions were normalized. Reactions performed with succinate at 4°C are shown (Ice). Alternatively, an exogenous ATP regeneration system, creatine kinase and its substrate creatine phosphate, was added in place of succinate, which was sufficient for fusion (NT [gray bars], succinate vs. creatine kinase/phosphate). Mitochondria under each condition were treated with complex III inhibitors (1 µM antimycin A or 0.4 µM myxothiazol) to produce ROS in the presence (C) or absence (D) of 4 mM GSH to drive the formation of GSSG. When respiration and ATP production were driven by succinate, fusion was inhibited under these conditions. The addition of exogenous ATP regeneration system rescued the inhibition of fusion inhibition caused by blocking complex III by antimycin A (Ant A) and myxothiazol (Myx). In the presence of exogenous ATP, fusion was activated upon complex III inhibition, as long as GSH was present (C). Reported values are the mean of three biological replicates, each performed in duplicate. Error bars are means ± SEM. (E) Extramitochondrial ROS activates fusion in a GSH-dependent manner. Mitochondria were incubated in the presence of increasing amounts of xanthine oxidase/xanthine, which produces ROS enzymatically. In the absence of GSH, mitochondrial fusion was not activated significantly by ROS (light gray). In the presence of 4 mM GSH, fusion was activated gradually, corresponding to increasing specific activity of xanthine oxidase (black). Reported values are the mean of three biological replicates, each performed in duplicate. Error bars are means ± SEM. (F) Fusion samples from E were processed for denaturing, nonreducing SDS-PAGE, followed by immunoblotting with anti-Mfn2 antibody (Abnova). One additional sample was included of mitochondria incubated with 1 mM GSSG as a positive control for the formation of Mfn2 disulfide oligomers. Statistical significance was analyzed using unpaired Student’s t test. *, P < 0.05; **, P < 0.01; n.s., not significant.