Figure 4.
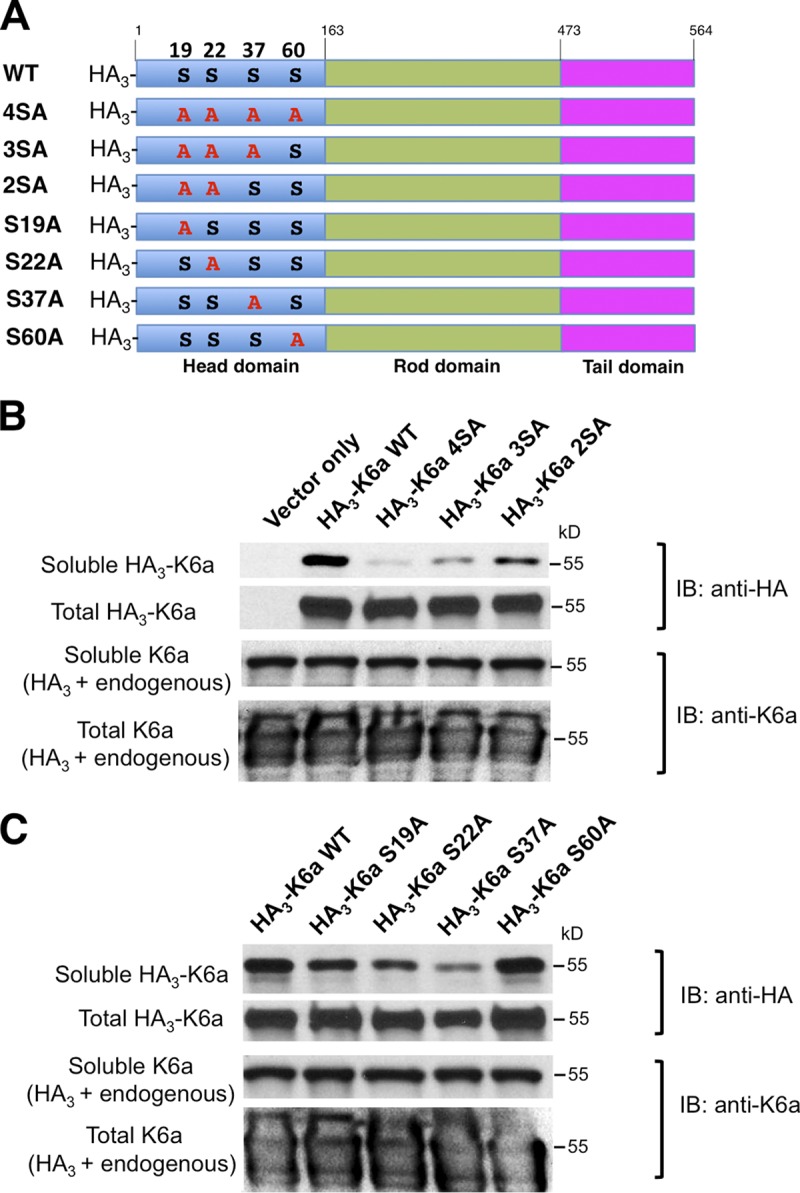
Site-specific serine-to-alanine K6a mutants are less soluble. (A) hTCEpi cells were nucleofected with empty plasmid or plasmids encoding HA-tagged WT K6a or HA-tagged K6a mutants with various Ser-to-Ala mutations. After harvest, cells were disrupted by NP-40 lysis buffer to dissolve soluble K6a or by RIPA buffer supplemented with 8 M urea to dissolve both soluble and filamentous K6a into total K6a. (B) Solubilities of K6a mutants with multiple serine-to-alanine mutations. Note that the solubilities of the quadruple (S19A, S22A, S37A, and S60A) and triple mutants (S19A, S22A, and S37A) were drastically reduced compared with WT, whereas the double mutant (S19A and S22A) had noticeable improvement. (C) Solubilities of K6a single serine-to-alanine mutants. Note that the single S19A, S22A and S37A mutants displayed markedly reduced solubilities compared with WT, with the S37A mutant being the least soluble. In contrast, the solubility of the S60A mutant was comparable to WT. Experiments were performed three times. IB, immunoblot.
