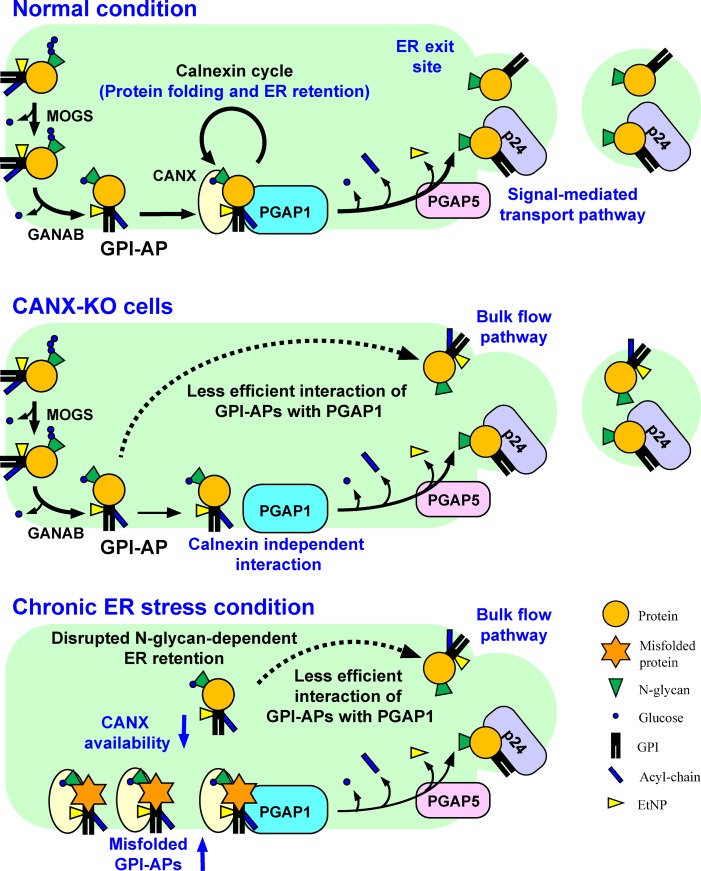
Figure 9.
Summary models for folding and processing of GPI-APs regulated by calnexin and ER homeostasis. Top: under normal conditions, N-glycans and GPI are transferred to newly synthesized GPI-APs. The glucose residues on N-glycans are trimmed by α-glucosidases I and II, resulting in a Glc1Man9GlcNAc2 structure, which is recognized by calnexin. Calnexin assists protein folding and facilitates GPI-inositol deacylation by PGAP1 through the temporal ER retention of GPI-APs. Calnexin also interacts with PGAP1 to increase the efficiency of the GPI-inositol deacylation. Once the protein portion of each GPI-AP is folded, calnexin dissociates from the GPI-AP, which is further remodeled by PGAP5 and efficiently incorporated into transport vesicles by a signal-mediated pathway involving p24 proteins. Middle: in CANX-KO cells, GPI-APs cannot retain in the ER and decrease the efficient interaction with PGAP1, resulting in transport of some GPI-APs without processing. Calnexin-independent GPI-inositol deacylation also exists. Bottom: under chronic ER stress conditions, misfolded GPI-APs accumulate in the ER and occupy the calnexin and PGAP1. Availability of calnexin and PGAP1 is therefore decreased, and normal GPI-APs without processing leak into transport vesicles through a bulk flow pathway, resulting in expression of inositol-acylated GPI-APs on the cell surface.