Figure 3.
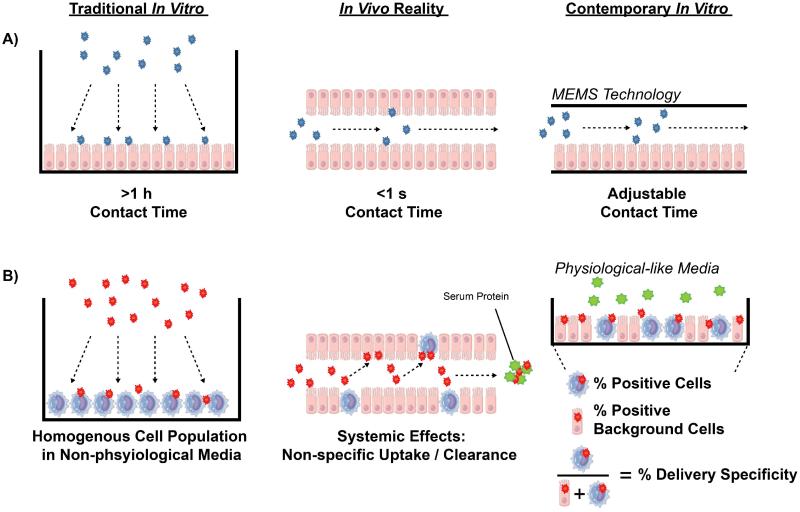
Physical design considerations of in vitro systems vs. in vivo conditions. Gene therapy studies typically incubate delivery transfection agents (red [A] and blue [B]) under conditions that are not representative of systemic in vivo circulation. One such condition is the use of long incubation times (A), which are physiologically unrealizable and can be avoided by using microelectromechanical systems (MEMS). Additionally, in vitro measurements of preferential cell uptake are usually validated using homogenous cell populations in media lacking relevant levels of potentially interfering serum proteins (green particles) (B). Transfection studies can be improved by determining delivery specificity metrics in physiological media with heterogeneous cell populations to accurately predict non-specific interactions with serum proteins and off-target delivery respectively.