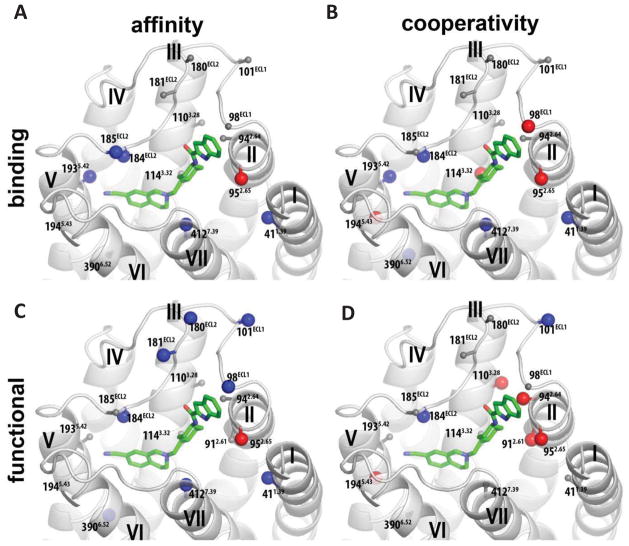
Figure 1.
A homology model of the D2R with SB269652 docked. Residues are highlighted for which mutation to alanine significantly alters the affinity of SB269652 (pKB, A) or cooperativity with [3H]spiperone (Logα, B) in a radioligand binding assay (A & B), or the affinity (pKB, C) of SB269652 and/or its cooperativity with dopamine (Logαβ, D) as determined in an assay measuring phosphorylation of ERK1/2 (C & D). Residues are highlighted for which there was significant increase (blue) or decrease (red) between parameter at wild type versus mutant D2R as determine by a one-way ANOVA with Dunnetts post-hoc test, P < 0.05 (Tables 3 & 4).