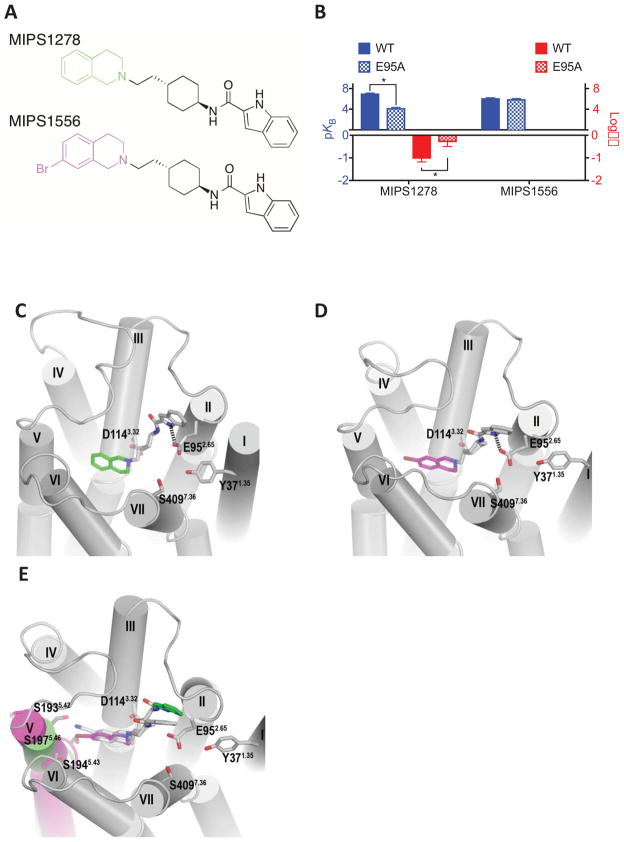
Figure 4.
(A) Derivatives of SB269652 in which the 7-cyano substitution of the THIQ moiety was replaced with hydrogen (MIPS1278) or bromine (MIPS1576). (B) In an assay measuring ERK1/2 phosphorylation MIPS1278 displayed allosteric pharmacology and a decreased affinity and cooperativity at the E952.65A mutant. The 7-bromo substituted derivative (MIPS1576) displayed pharmacology best fit by a competitive model at the wild type receptor and was insensitive to the E952.65A mutation. Data represents the mean ± SD of four independent experiments. * P < 0.05 between parameter at wild type versus E952.65A D2R as determine by unpaired two-tailed Student’s test. Molecular modelling and docking experiments revealed that the indolic NH of both MIPS1278 (C) and MIPS1556 (D) were predicted to participate in a hydrogen bond with E952.65A. (E) Comparison of the D2R when either MIPS1278 and MIPS1556 were bound revealed that the larger 7-Br substitution of MIPS1556 caused a distortion in TM5.