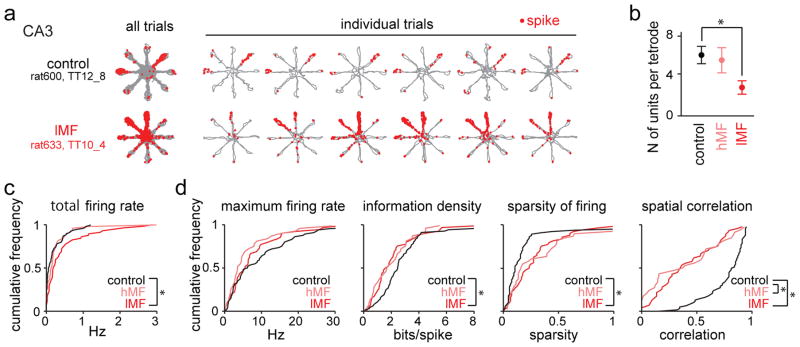
Figure 6. Place-specific firing of CA3 pyramidal neurons.
(a) Each row is a representative example of place-specific firing of a CA3 pyramidal cell (top, control; bottom, DG-lesion) in the spatial WM task. Similar results were obtained in 116 control cells from 4 animals and in 57 lMF cells from 9 DG-lesioned rats and are quantified in panels (c) and (d). For each neuron, the trajectories (gray) with spike locations (red dots) are shown superimposed for all 20 trials in a recording session and separately for the first 6 trials (from left the right). CA3 place fields in control animals were frequently restricted to a single arm, but firing fields were broader at recording sites of DG-lesioned rats (see Supplementary Fig. 14 for additional examples). (b) Average number of CA3 principal cells that were recorded from each tetrode. Fewer CA3 neurons were detected at lMF sites, compared to CA3 recording sites from control rats and hMF sites from DG-lesioned rats (n = 17, 10, and 20 in control, hMF, lMF sites in 4, 5, and 9 animals, respectively; control vs hMF, U = 250.5, Z = 0.61, P > 0.99; control vs lMF, U = 443.5, Z = 3.70, P = 4.2 × 10−4, two-sided Mann-Whitney U test followed by posthoc Bonferroni corrections). * P < 0.05. Error bars are SEM. (c) Cumulative distribution of total firing rates for the entire sample of CA3 cells (n = 116, 48, and 57 cells from 4, 5, and 9 animals in control, hMF, and lMF groups, respectively). The firing rates of CA3 neurons were selectively increased at sites with low density MF input (lMF) (control vs hMF, Dmax = 0.20, P = 0.11; control vs lMF, Dmax = 0.30, P = 0.0024, Kolmogorov-Smirnov test with Bonferroni’s correction). * P < 0.05. (d) Cumulative distribution of maximum in-field firing rates, information density scores, sparsity of firing, and spatial correlation for CA3 cells recorded from control, hMF, and lMF sites (n = 64, 34, and 47 cells from 4, 5, and 9 animals, respectively). Spatial measurements were calculated from all cells with an average firing rate > 0.05 Hz. The precision of spatial firing was reduced in DG-lesioned rats, and the effect was more pronounced at lMF sites (maximum firing rate, control vs hMF, Dmax = 0.26, P = 0.16; control vs lMF, Dmax = 0.16, P = 0.84; information density, control vs hMF, Dmax = 0.26, P = 0.15; control vs lMF, Dmax = 0.30, P = 0.022; sparsity, control vs hMF, Dmax = 0.30, P = 0.051; control vs lMF, Dmax = 0.35, P = 0.0030). However, reliability of spatial firing was reduced irrespective of MF density (spatial correlation, control vs hMF, Dmax = 0.56, P = 3.2 × 10−6; control vs lMF, Dmax = 0.55, *P = 1.4 × 10−7, Kolmogorov-Smirnov test with Bonferroni’s correction). * P < 0.05.