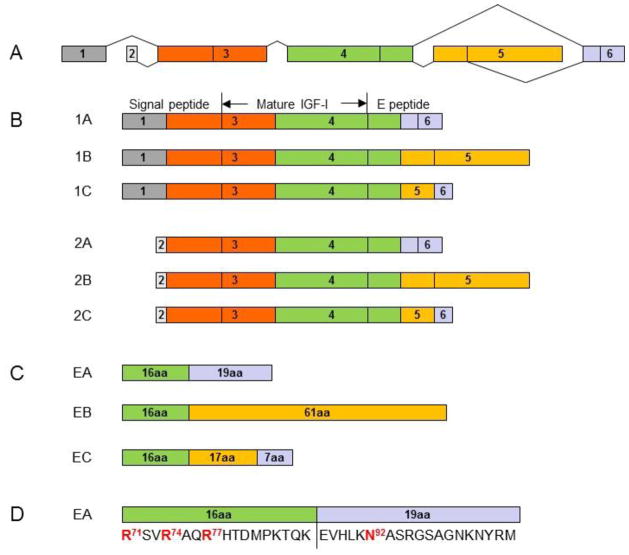
Figure 1.
Alternative splicing of the human IGF-I gene. The human IGF-I gene has six exons (A). Alternative splicing of the gene gives rise to six IGF-I mRNA variants for six corresponding IGF-I prepropeptides (1A, 1B and 1C, and 2A, 2B and 2C) in two classes and three groups (B). Classes 1 and 2 variants arise from exon 1 and exon 2, respectively. Group A variants exclude exon 5 while group B variants exclude exon 6. Group C variants produced by an internal splice site within exon 5, which causes a frame shift and premature termination in exon 6. Each of IGF-I prepropeptides consists of an N-terminal signal peptide, mature peptide and a C-terminal E peptide. Proteolytic processing at both N- and C-terminal ends of the IGF-I prepropeptides produces the same 70 amino acid mature IGF-I peptide. The removal of signal peptides from the 6 IGF-I prepropeptides results in 3 IGF-I propeptides (proIGF-IA, proIGF-IB and proGF-IC), each containing a different E peptide (EA peptide, EB peptide or EC peptide) in addition to the mature IGF-I peptide. The EA peptide (35 amino acids), EB peptide (77 amino acids) and EC peptide (40 amino acids) share the first 16 amino acid sequence from Exon 4 (C). The three Arg (Arg71, Arg74 and Arg77) in the 16 amino acid sequence are involved in the processing of proIGF-IA to produce the mature IGF-I. Only the EA peptide contains a putative N-glycosylation site, Asn92 in the 19 amino acid region from Exon 6 (D).