Abstract
Matrix metalloprotease-3 (MMP3) activation mediates the tissue plasminogen activator (tPA)-induced hemorrhagic transformation (HT) after stroke. Hyperglycemia (HG) further exacerbates this outcome. We have recently shown that HG increases MMP3 activity in the brain after stroke. However, the combined HG-tPA effect on MMP3 activation, and the mechanisms through which MMP3 is activated were not previously reported. Accordingly, this study tested the hypothesis that tPA and HG increases MMP3 activity in the brain after stroke through peroxynitrite induced tyrosine nitration. Normoglycemic and mildly hyperglycemic male Wistar rats were subjected to middle cerebral artery suture occlusion for 90 minutes or thromboembolic occlusion, and up to 24 h reperfusion, with and without tPA. MMP3 activity and tyrosine nitration were evaluated in brain homogenates at 24 h. Brain microvascular endothelial cells (BMVEC) were subjected to either 3 h hypoxia or 6 h OGD under either normal or high glucose conditions with or without tPA, with or without peroxynitrite scavenger, FeTPPs. MMP3 activity and MMP3 tyrosine nitration were assessed at 24 h. HG and tPA significantly increased activity and tyrosine nitration of MMP3 in the brain. In BMVECs, tPA but not HG increased MMP3 activity. Treating BMVEC with FeTPPs significantly reduced the tPA-induced increase in MMP3 activity and nitration. Augmented oxidative and nitrative stress may be potential mechanisms contributing to MMP3 activation in hyperglycemic stroke, especially with tPA administration. Peroxynitrite may be playing a critical role in mediating MMP3 activation through tyrosine nitration in hyperglycemic stroke.
Keywords: Hyperglycemia, Tissue plasminogen activator, Matrix metalloprotease 3, Peroxynitrite, Hemorrhagic transformation
1. Introduction
Tissue plasminogen activator (tPA) increases the risk of cerebral hemorrhage after stroke, and the incidence of hyperglycemia (HG) further exacerbates this outcome [1–4]. We have recently shown that the combination of HG and tPA exacerbates hemorrhagic transformation and worsens functional outcomes after stroke more than each of them alone [5]. However, the underlying mechanism through which this injury occurred was not known. We also showed that acute HG increased matrix metalloprotease 3 (MMP3) activity in the brain after stroke and this was associated with exacerbated cerebral hemorrhage and worse functional outcomes [6]. It was previously shown that MMP3, but not MMP9, plays a critical role in mediating the tPA-induced cerebral hemorrhage after stroke [7]. However, the impact of the interaction between HG and tPA on MMP3 activation after stroke, and the underlying mechanism through which HG-tPA induces MMP3 activation was not studied.
Peroxynitrite is a potent oxidizing and nitrating agent that can cause lipid peroxidation and protein nitration and consequently can be involved in blood brain barrier (BBB) disruption. Peroxynitrite is increased in the brain after the ischemic injury and HG augments this effect [8,9]. It was recently shown that peroxynitrite plays a role in MMP3 activation through tyrosine nitration in the synovial fluid of patients with temporomandibular joint (TMJ) disorders [10]. However, it is not known whether this is the mechanism through which MMP3 is activated in the brain in hyperglycemic stroke. Accordingly, this study was designed to test the hypothesis that: 1) The interaction between tPA and HG increases MMP3 activity in the brain after stroke; and 2) Peroxynitrite-induced MMP3 nitration is the underlying mechanism of increased MMP3 activity in the brain in hyperglycemic stroke.
Materials and Methods
Animal Models and Study Groups
The animals were housed at the Augusta University animal care facility, which is approved by the American Association for Accreditation of Laboratory Animal Care. This study was conducted in accordance with the National Institute of Health guidelines for the care and use of animals in research and all protocols were approved by the institutional animal care and use committee.
Study 1. Determine the effect of HG and tPA on MMP3 activity
Study 1.1
Determine the effect of HG and tPA on MMP3 activity in vivo in hyperglycemic stroke.
Control/Normoglycemic (NG) or mild HG (140 – 200 mg/dl) male Wistar rats (320 g) were subjected to either 90 minutes suture or thromboembolic middle cerebral artery occlusion and up to 24 h reperfusion. Animals were randomized to receive either tPA (Cathflo Activase (Alteplase), Genentech) or vehicle (water for injection) of equal volume. tPA (1mg/kg) was intravenously infused over 20 minutes through the jugular vein 2h after induction of ischemia to rats with either suture or thromboembolic occlusion. At 24h, animals were sacrificed, brains were isolated and homogenized and examined for the MMP3 activity.
Study 1.2
Determine the effect of HG and tPA on MMP3 activity in brain microvascular endothelial cells (BMVEC).
Primary BMVEC were isolated from the brains of Wistar rats by immunomagnetic separation using Dynabeads as previously described [11]. BMVEC were cultured in DMEM and incubated in hypoxic chamber under low oxygen tension for 3 hours and then further cultured in DMEM with normal glucose (NG = 5 mM) or high glucose (HG = 25 mM) for 22.5 hours to mimic the in vivo model. Study groups are: 1) Normal glucose (NG); 2) NG + tPA; 3) High glucose (HG); 4) HG + tPA. Control cells were cultured in DMEM with normal or high glucose for 24 hours under normoxia. tPA was added (10 µg/ml) at the end of 3h hypoxia or normoxia. At the end of the incubation period (24 h), supernatant and cell lysates were collected separately and MMP3 activity was measured.
Study 2. Determine the role of peroxynitrite in activating MMP3 through tyrosine nitration in hyperglycemic stroke
Study 2.1. In vivo
Brain homogenates from study 1.1 were analyzed for MMP3 nitration by immunoprecipitation.
Study 2.2. In vitro
BMVEC homogenates from study 1.2 were analyzed for MMP3 nitration. Furthermore, additional set of cells were subjected to oxygen glucose deprivation (OGD) for 6 hours and treated with either tPA alone, FeTPPs (5,10,15,20-Tetrakis(4-sulfonatophenyl) porphyrinato Iron (III), Chloride, 2.5 ug/ml) (peroxynitrite decomposition catalyst) alone or a combination of both tPA and FeTPPs. Treatments were added at the end of 6 h OGD, cells were harvested at 24 h and tested for MMP3 activity and MMP3 tyrosine nitration.
Stroke Surgery
Focal cerebral ischemia was performed as previously described [12]. Briefly, a midline cervical incision was made to expose the common carotid artery. The external carotid artery (ECA) was separated, ligated and cauterized. An arteriotomy was performed on the ECA stump. A rounded-tip 3-0 monofilament nylon suture (prepared carefully under microscope with high magnification power to ensure uniformity) was inserted into the ECA stump and advanced through the internal carotid artery to occlude the origin of MCA. For the embolic model, the surgical procedure remained the same as in suture model except that instead of the monofilament suture, a PE-10 catheter containing the clot was inserted through the ECA stump to deliver the clot to the origin of the MCA. Scanning laser Doppler (Pim-3, Perimed, ST) was used to confirm a similar degree of drop in CBF among groups. The percent drop in CBF after stroke was determined by comparing to baseline [13]. Animals with reduction in CBF less than 40% from baseline were excluded.
Preparation of Blood Clots
Fresh blood clots were prepared as we previously reported in details [14,15,5].
Evaluation of MMP3 Enzymatic Activity
The enzymatic activity of MMP-3 was determined using a fluorescence resonance energy transfer (FRET) peptide and immunocapture assay as previously described elsewhere with minor modifications [16]. Briefly, 50 µg total protein of homogenized brain tissue were incubated at 4°C for 2 h with rabbit polyclonal anti-MMP-3 antibody (Cat.No. sc-6839-R; Santa Cruz Biotechnology, Dallas, TX, USA). A/G agarose beads were then added and allowed to incubate overnight at 4°C. The beads were then washed and samples are transferred to black 96 well plate and 100 ul of 2mM 5-FAM/QXL™520 FRET peptide (Cat. No. 60580-01; AnaSpec, San Jose, CA, USA) in assay buffer were added per well. Plates were incubated for 8 h at 37°C and the relative fluorescence units (RFUs) were read and monitored at excitation/emission wavelengths of 485/528 nm in a Synergy HT Multi-mode microplate fluorescence reader (BioTek, Winooski, VT, USA) running Gen5™ data analysis software.
Immuno-precipitation and Western Blotting
MMP3 nitration was evaluated by immuno-precipitation (IP) of either rats’ brain homogenates or BMVEC lysates for MMP3 using anti-MMP3 antibody followed by western blotting against nitrated proteins using anti-nitrotyrosine antibody. Briefly, 100 ug protein from either brain homogenates or BMVEC lysates were incubated with anti-MMP3 antibody (Cat.No. sc-6839-R; Santa Cruz Biotechnology, Dallas, TX, USA) for 4 h at 4°C. A/G agarose beads were then added and allowed to incubate overnight at 4°C. On the next day, anti-MMP3-A/G protein complex was washed 2 times, precipitated by centrifugation and then analyzed by Western blotting using anti-nitrotyrosine antibody. 50 ul of 2× loading buffer was added to the immune-precipitated protein-beads complex and boiled for 20 minutes then centrifuged and equal volumes of the supernatant (30 ul) were loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. MMP3 nitration was determined by using anti-nitrotyrosine antibody (1:500) (05-233, Millipore, USA). Primary antibodies were detected using horseradish peroxidase-conjugated antibody and enhanced chemiluminescence. Band intensity was quantified by densitometry software (Alpha Innotech; Santa Clara, CA). Western blotting for MMP3 in BMVEC was done the same way using anti MMP3 antibody (1:500) (ab53015, Abcam, USA).
Statistical Analysis
The effect of hyperglycemia and tPA treatment on MMP3 activity was assessed using a 2 HG (no vs. yes) by 2 tPA (no vs. yes) ANOVA where a significant interaction would indicate a differential effect of tPA on MMP3 activity dependent on HG status. The effect of tPA on MMP3 activity in BMVEC compared to untreated cells was determined using a unpaired Student's t-test. The effect of HG on MMP3 tyrosine nitration (OD) compared to NG groups was determined using a student unpaired t-test. The effect of HG and hypoxia treatment on MMP3 tyrosine nitration in BMVEC was assessed using a 2 HG (no vs. yes) by 2 hypoxia (no vs. yes) ANOVA where a significant interaction would indicate a differential effect of hypoxia on MMP3 activity dependent on HG status. Effect of FeTTPs treatment was analyzed by one-way ANOVA within NG or HG groups. Statistical significance was determined at p<0.05. Tukey’s or Bonferroni post-hoc tests were used to compare means from significant one way or two-way ANOVAs, respectively.
Results
The Effect of HG and tPA on MMP3 Activity and Nitration In Vivo
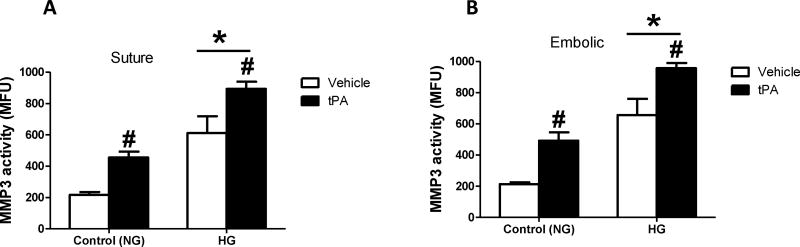
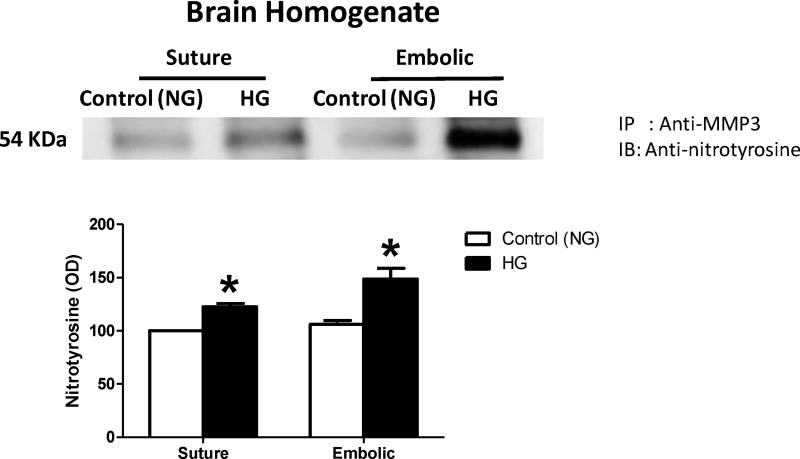
HG significantly increased MMP3 activity in ischemic hemisphere homogenates compared to NG animals. The administration of tPA significantly increased total brain MMP3 activity compared to non-treated animals. There was no interaction between HG and tPA interventions (Fig. 1A&B). The HG-induced MMP3 activation was associated with significant increase in MMP3 tyrosine nitration in the brain after stroke, in rats subjected to either suture or thromboembolic occlusion (Fig. 2).
Figure 1. HG and tPA significantly increased MMP3 activity in the brain after stroke.
HG and tPA treatment significantly increased MMP3 activity in the ischemic hemispheres in rats subjected to either suture (A) or thromboembolic occlusion (B). *p<0.0001 vs control (NG), #p<0.0001 vs vehicle (non-tPA), n=6/group. Panel A, interaction F(1,38)=0.1537, p=0.6972; *HG F(1,38)=53.32; #tPA F(1,38)=20.82. Panel B, interaction F(1,37)=0.04094, p=0.84; *HG F(1,37)=78.26; #tPA F(1,37)=31.8.
Figure 2. HG significantly increased MMP3 tyrosine nitration in the brain after stroke.
HG significantly increased MMP3 tyrosine nitration in the brain after stroke in rats subjected to either suture or thromboembolic occlusion. *p<0.01 vs control (NG), n=6/group. IP; Immunoprecipitation, IB; Immunoblotting.
The Effect of HG and tPA on MMP3 Activity and MMP3 Nitration In Vitro
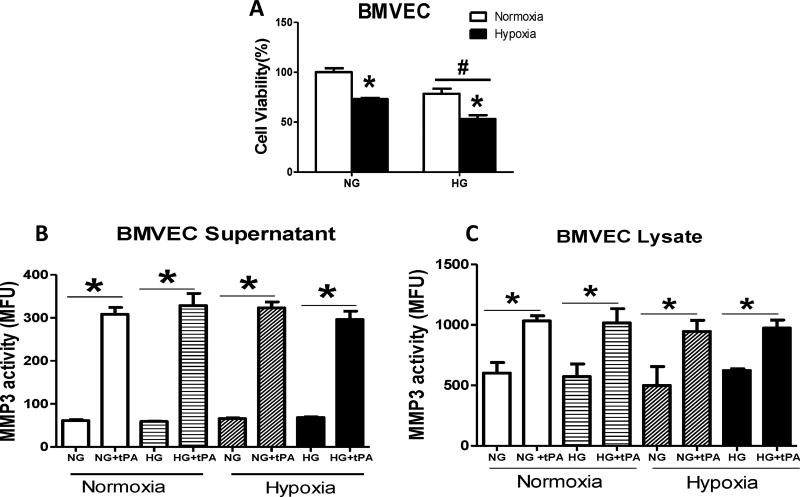
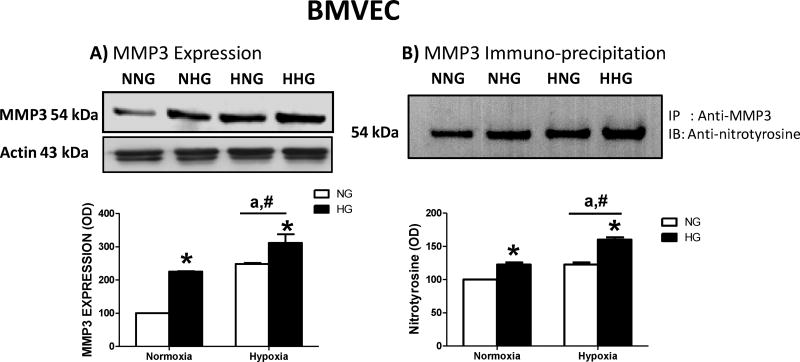
The administration of tPA significantly increased MMP3 activity in BMVEC under normal or HG conditions, whether these cells were subjected to either normoxia or hypoxia. This effect was detected in both BMVEC lysate and supernatant (Fig. 3A&B). High glucose or hypoxia alone significantly increased MMP3 expression (Fig. 4A) and MMP3 nitration (Fig. 4B) in BMVECs. There was a treatment/intervention interaction such that hypoxia induced MMP3 expression in NG but not HG group (Fig. 4A). On the other hand, hypoxia mediated nitrotyrosine formation in HG but not NG group (Fig. 4B).
Figure 3. tPA significantly increased MMP3 activity in BMVECs after under normoxic and hypoxic conditions.
(A) Hypoxia and HG reduced cell viability (#p=0.0007 vs NG, *p<0.0001 vs normoxia). tPA significantly increased MMP3 activity in BMVECs under normal or high glucose conditions whether cells were subjected to normoxia or hypoxia. This effect was detected in both BMVEC supernatant (B) and lysate (C). *p<0.0001 vs non-tPA, n=4/group.
Figure 4. HG increased MMP3 expression and MMP3 tyrosine nitration in BMVEC in normoxic and hypoxic conditions.
(A) Both high glucose and hypoxia alone significantly increased MMP3 expression in BMVECs. Hypoxia-induced increase in MMP3 expression was significant in NG but not HG group indicating a treatment and intervention interaction. *HG p=0.0001, F(1,8)=49.19, #hypoxia p<0.0001 F(1,8)=76.64, ainteraction p=0.0495, F(1,8)=5.35. (B) While both condition also increased MMP3 nitration, interaction suggested that hypoxia increased nitrotyrosine levels in HG but not NG group. *HG p<0.0001, F(1,12)=108, #hypoxia p<0.0001, F(1,12)=108, ainteraction p=0.0232, F(1,8)=6.75. n=4/group. IP; Immunoprecipitation, IB; Immunoblotting. [NNG= normoxia normal glucose; NHG= normoxia high glucose; HNG= hypoxia high glucose; HHG= hypoxia high glucose].
The Effect of Peroxynitrite Inhibition on MMP3 Activity
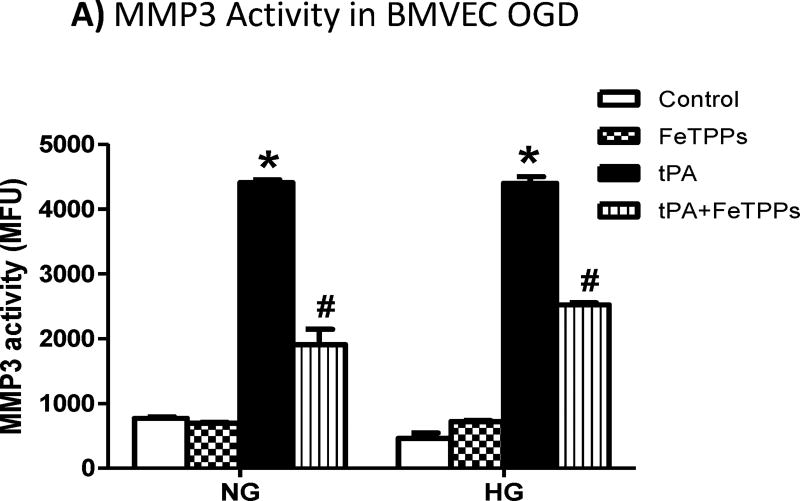
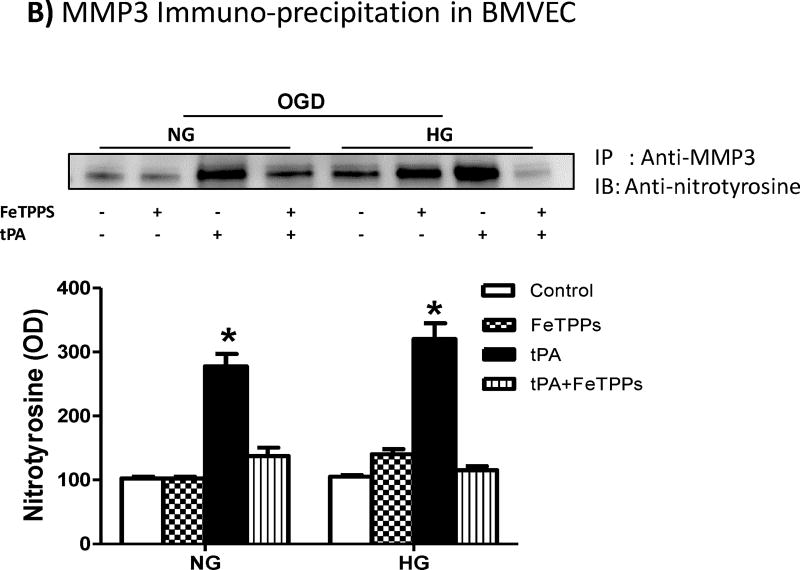
The use of tPA significantly increased MMP3 activity (Fig. 5A) and MMP3 tyrosine nitration (Fig. 5B) in BMVECs subjected to OGD under either NG or HG conditions. The use of FeTPPs significantly reduced but not restored MMP3 activity (Fig. 5A), although FeTTPs treatment almost completely prevented nitrotyrosine formation (Fig. 5B).
Figure 5. Peroxynitrite inhibition significantly reduced tPA-induced MMP3 activation in BMVEC after OGD.
The use of tPA significantly increased (A) MMP3 activity in BMVEC subjected to OGD under normal or high glucose conditions and this was associated by significant increase in (B) MMP3 tyrosine nitration. The use of the peroxynitrite decomposition catalyst, FeTPPs, significantly reduced the tPA-induced increase in MMP3 activity and MMP3 nitration. *p<0.0001 vs other groups, #p<0.0001 vs control or FeTPPs, n=4/group. IP; Immunoprecipitation, IB; Immunoblotting.
Discussion
Matrix metalloproteases, especially MMP2 and MMP9, play a major role in mediating HT after stroke through degradation of the tight junction proteins and disruption of the BBB [17]. MMP3 is a zinc endopeptidase that has a broad substrate specificity and can target and degrade all the component of the neurovascular unit [18]. It was previously shown that MMP3 plays a critical role in mediating the tPA induced intracranial bleeding in mice after stroke [7]. We recently showed that the HG-induced MMP3 activation exacerbated the hemorrhagic transformation and worsened the functional outcomes after stroke [6]. However, it was not known how MMP3 gets activated in hyperglycemic stroke. Furthermore, we also showed in an earlier study that the interaction between tPA and HG significantly increased HT and worsened stroke outcomes [5], but the underlying mechanism was not known. So, in order to connect all the threads together, this study was designed to test the hypothesis that; 1- The interaction between HG and tPA increases MMP3 activity after stroke, and 2- MMP3 activation is mediated through peroxynitrite induced MMP3 tyrosine nitration.
Current findings show that either HG or tPA alone increases MMP3 activity in the brains of rats subjected to either suture or embolic occlusion. While the effect of the combination of HG and tPA on MMP3 activity is not synergistic, both interventions significantly affected MMP3 expression and activity. Correlating this to our previous findings, we propose that MMP3 plays an important role in mediating the HG-tPA induced HT and worse functional outcomes after stroke.
We found that administration of tPA increased the activity of MMP3 in BMVECs isolated from rat brain, whether these cells were cultured under normal or high glucose conditions, subjected to either normoxia or hypoxia. However, In contrast to our hypothesis and in vivo findings, we did not see a further increase in MMP3 activity with hypoxia or high glucose alone in BMVECs. This may be because the insult was not strong enough to trigger this effect, as we subjected the cells to only 3 hours of hypoxia and cultured in high glucose for only 18 h. In 2009, Suzuki et al showed that administration of tPA has increased the production of MMP3 in brain endothelial cells derived from mouse brain subjected to 6 hours of OGD. This effect was not seen when the cells were subjected to only 3 h of OGD [19]. However, when we further subjected the cells to 6 h of OGD, we still did not see an increase in MMP3 activity with high glucose compared to normal glucose. It is possibly that we either did not achieve the hypoxia threshold necessary to trigger MMP3 activation, or the elevated MMP3 activity that we see in total brain homogenates is a result of upregulation of endogenous in vivo enzymatic activity that is not available in the cultured endothelial cells and requires interaction of other cell types. This can also explain why we have seen an increase in MMP3 protein expression that was not associated with an increase in activity. Other explanation may be that endogenous MMPs inhibitors, like tissue inhibitors of metalloproteases (TIMPs), were suppressed or restrained in the brain environment, which was not the case in the cell culture system.
The underlying mechanisms through which HG worsens the neurovascular injury after stroke were always attributed to the HG-induced production of reactive oxygen/nitrogen species, especially superoxide anions and peroxynitrite [8]. Given that HG and stroke increase oxidative stress, we hypothesized that peroxynitrite may be increasing MMP3 activity in hyperglycemic stroke through induced tyrosine nitration. In 2009, Fujita et al showed that MMP3 activation takes place through tyrosine nitration in the synovial fluid of patients with TMJ disorders, and thus leading to the pathophysiological progression of the internal derangement (ID) of TMJ [10]. Our immuno-precipitation studies showed that HG increased MMP3 nitration in the brains of rats subjected to either suture or thromboembolic occlusion. This increase in MMP3 tyrosine nitration corresponded to an increase in MMP3 activity. However, this was not the case in BMVECs, as cells subjected to hypoxia combined with high glucose showed an increase in MMP3 expression and MMP3 tyrosine nitration without a corresponding increase in activity. These findings show that tyrosine nitration may be one but not the only underlying mechanism causing MMP3 activation in hyperglycemic stroke. This is further supported by our in vivo data in which the combination effect was not synergistic suggesting that there may be more than one mechanism involved. The administration of tPA has significantly increased the activity of MMP3 in BMVECs and this corresponded to significant increase in MMP3 tyrosine nitration. Interestingly, the use of the peroxynitrite decomposition catalyst, FeTPPs, has significantly reduced MMP3 tyrosine nitration and MMP3 activity, further supporting our hypothesis that peroxynitrite-mediated nitration contributes to MMP3 activation. By the same token, the fact that MMP3 activity is not completely restored with FeTPPs suggests that additional mechanisms other than tyrosine nitration are involved in MMP3 activation. Previously we showed that FeTPPs administration at reperfusion significantly reduced MMP9 activity in Goto-Kakizaki diabetic rats and this was associated with reduced HT [20]. This raises the question for further future investigations, whether FeTPPs or a nitration inhibitor given at reperfusion would prevent MMP3 activation and improve outcomes in hyperglycemic stroke.
In conclusion, this study is novel to show that augmented MMP3 activity in the brain especially under HG conditions or with tPA treatment involves peroxynitrite-induced tyrosine nitration. In correlation with our previous findings, this identifies peroxynitrite and MMP3 as critical mediators of the HG-tPA induced HT, and points them out as potential therapeutic targets in hyperglycemic stroke.
Acknowledgments
Adviye Ergul is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award (BX000347), VA Research Career Scientist Award, and NIH (R01NS083559) to Adviye Ergul; VA Merit Award (BX000891) and NIH award (NS063965) to Susan C. Fagan, and American Heart Association Predoctoral Fellowship (13PRE17090026) to Sherif Hafez. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- tPA
Tissue plasminogen activator
- MMP3
Matrix metalloprotease 3
- HT
Hemorrhagic transformation
- HG
Hyperglycemia
- NG
Normoglycemia
- MCA
Middle cerebral artery
- BMVEC
Brain microvascular endothelial cells
- FeTPPs
5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron (III) Chloride
- BBB
Blood brain barrier
- TMJ
Temporomandibular joint
- ECA
External carotid artery
- CBF
Cerebral blood flow
- FRET
Fluorescence resonance energy transfer
- IP
Immuno-precipitation
- IB
Immunoblotting
Footnotes
Conflict of Interest
Sherif Hafez declares that he has no conflict of interest.
Mohammed Abdelsaid declares that he has no conflict of interest.
Susan C. Fagan declares that she has no conflict of interest.
Adviye Ergul declares that she has no conflict of interest.
Compliance with Ethics Requirements
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Fugate JE, Rabinstein AA. Update on intravenous recombinant tissue plasminogen activator for acute ischemic stroke. Mayo Clin Proc. 2014;89(7):960–972. doi: 10.1016/j.mayocp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39(2):384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 3.Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59(5):669–674. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 5.Hafez S, Hoda MN, Guo X, Johnson MH, Fagan SC, Ergul A. Comparative Analysis of Different Methods of Ischemia/Reperfusion in Hyperglycemic Stroke Outcomes: Interaction with tPA. Transl Stroke Res. 2015;6(3):171–80. doi: 10.1007/s12975-015-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafez S, Abdelsaid M, El-Shafey S, Johnson MH, Fagan SC, Ergul A. Matrix Metalloprotease 3 Exacerbates Hemorrhagic Transformation and Worsens Functional Outcomes in Hyperglycemic Stroke. Stroke. 2016;47(3):843–851. doi: 10.1161/STROKEAHA.115.011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5(8):1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- 8.Bemeur C, Ste-Marie L, Montgomery J. Increased oxidative stress during hyperglycemic cerebral ischemia. Neurochem Int. 2007;50(7–8):890–904. doi: 10.1016/j.neuint.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Lehner C, Gehwolf R, Tempfer H, Krizbai I, Hennig B, Bauer HC, Bauer H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid Redox Signal. 2011;15(5):1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita H, Morisugi T, Tanaka Y, Kawakami T, Kirita T, Yoshimura Y. MMP-3 activation is a hallmark indicating an early change in TMJ disorders, and is related to nitration. Int J Oral Maxillofac Surg. 2009;38(1):70–78. doi: 10.1016/j.ijom.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes. 2012;61(6):1533–1542. doi: 10.2337/db11-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W, Kozak A, El-Remessy A, Johnson M, Pillai B, Fagan S. Acute Treatment with Candesartan Reduces Early Injury After Permanent Middle Cerebral Artery Occlusion. Transl Stroke Res. 2011;2:179–85. doi: 10.1007/s12975-010-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova MA, Johnson MH, Ergul A, Hill WD, Hess DC, Sazonova IY. Sex-independent neuroprotection with minocycline after experimental thromboembolic stroke. Exp Transl Stroke Med. 2011;3(1):16. doi: 10.1186/2040-7378-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Qu Z, Prakash R, Chung C, Ma H, Hoda MN, Fagan SC, Ergul A. Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res. 2013;1541:106–114. doi: 10.1016/j.brainres.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42(5):1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38(3):376–385. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EM, Hwang O. Role of matrix metalloproteinase-3 in neurodegeneration. J Neurochem. 2011;116(1):22–32. doi: 10.1111/j.1471-4159.2010.07082.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114(15):3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 20.Kelly-Cobbs AI, Prakash R, Li W, Pillai B, Hafez S, Coucha M, Johnson MH, Ogbi SN, Fagan SC, Ergul A. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304(6):H806–815. doi: 10.1152/ajpheart.00720.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]