Abstract
Numerous studies have reported that temporal order perception is biased in neurological patients with extinction and neglect. These individuals tend to perceive two objectively simultaneous stimuli as occurring asynchronously, with the ipsilesional item being perceived as appearing prior to the contralesional item. Likewise, they report that two stimuli occurred simultaneously in situations where the contralesional item is presented substantially prior to the ipsilesional item. Therefore, they exhibit a biased point of subjective simultaneity (PSS). Here we demonstrate that the magnitude of this effect is modulated by the relative position of the stimuli with respect to the patient’s trunk. This effect was only observed in patients who still exhibited neglect symptoms, and neither the pathological bias nor substantial modulation were observed in individuals who had recovered from neglect, those who never had neglect or neurologically healthy controls. Crucially, our design kept the retinal and head-centered coordinates of these stimuli constant, providing a pure measure for the influence of egocentric trunk position. This finding emphasizes the interaction of egocentric spatial position on the temporal symptoms observed in these individuals.
Keywords: Prior entry, visual attention, spatial neglect, extinction, human visual perception, stroke, human
Introduction
Biased spatial perception is a common consequence of predominantly right hemisphere brain injury. For example, visual spatial neglect is a syndrome where individuals are impaired at detecting stimuli on their contralesional side. A popular test for neglect is cancellation where individuals are asked to find all occurrences of the letter ‘A’ on a piece of paper cluttered with characters. People with neglect tend to only find the targets on the ipsilesional side of the page. One well established finding is that this exploration and attentional orienting performance tends to be modulated by trunk position (for review Karnath, 2015), such that more targets are found if the paper is shifted ipsilesionally with respect to trunk position. On the other hand, task performance is not similarly influenced by head position or initial gaze position (Karnath et al., 1991, 1993). While this syndrome is defined by this spatial deficit, previous work has suggested that many individuals with perceptual deficits following stroke also experience problems in temporal processing (for review, see Becchio and Bertone, 2006). For example, in the ‘temporal order judgment task’ when observing two objectively simultaneous items, individuals with extinction and neglect tend to perceive the events as asynchronous, with the contralateral item reported as appearing first (Rorden et al., 1997). However, the relationship between these spatial and temporal deficits remains largely unexplored. One possibility is that the temporal deficits are completely independent of the spatial deficits, and associations between these symptoms merely reflect that the neighboring spatial and temporal modules are often injured together. This notion is consistent with claims that the human brain has a dedicated ‘when’ system (which resolves the temporal sequence of events) in addition to the well established ventral ‘what’ (object identification) and dorsal ‘where’ (stimuli location) systems (Battelli et al., 2007; Battelli et al., 2008). Alternatively, it is logically possible that these temporal deficits are a consequence of the core spatial disorder. According to this view, contralesional stimuli are under-represented in these patients (Driver and Pouget, 2000), and therefore responses are weaker and take longer to reach the threshold required for detection. These two competing models make testable predictions. Specifically, if temporal and spatial deficits are independent, we should see similar temporal deficits regardless of spatial perception. On the other hand, if the temporal deficit is an emergent property of the spatial deficit, the temporal deficits should by modulated by spatial location. Our goal was to directly test this prediction using the popular temporal order task.
According to an integrated view of the temporal and perceptual deficits seen in spatial neglect, these patients suffer from a continuous rather than categorical spatially-modulated impairment of perceptual capacity (Driver and Pouget, 2000): they do not merely have a ‘good’ and ‘bad’ visual half-field, but rather a gradient from relatively intact perception in the ipsilesional field to a weaker representation in contralesional field. According to this model, contralesional stimuli generate weaker signals than ipsilesional competitors, and therefore require longer to reach a threshold sufficient to be perceived (Desimone and Duncan, 1995). This would explain the explicit neglect for relatively contralesional items as well as their delayed perception (reflecting the weaker signal). Therefore, one of the predictions of this unified model is that all the deficits associated with these syndromes become less severe for information on the right side of the space.
However, any explanation of the spatial deficits observed in neglect needs to define the frame of reference that defines the contralesional side: in theory this could be based on eye position, head position, trunk position, object-based position, or any combination of these (potentially modulated by gravitational direction). Here we leverage the fact that prior studies have emphasized that the core spatial deficit observed in neglect appears to be dominated by the stimuli’s position relative to the observer’s trunk, rather than with respect to the location of visual fixation or head position (for review Karnath, 2015). In fact, this is fortunate, as retinal eccentricity is well known to influence temporal processing, as we discuss later.
Individual differences in determining the temporal sequence of events have been explored for years. For example, astronomers measured the ‘personal equation’ to account for temporal biases that differ between observers (Spence and Parise, 2010). One popular task is the ‘temporal order judgment’ (TOJ) paradigm, where an observer is asked to report the sequence of events. This task is analogous to the job of a baseball umpire who needs to determine the temporal sequence of distant visual events, e.g. determining whether the batter’s foot touched the base before or after the ball touched the catcher’s glove. Studies of the TOJ have revealed that an individual’s point of subjective simultaneity (PSS, where an observer does not reliably report one item occurring before another) can be influenced by bottom-up (reflexive) as well as top-down (strategic) attentional cues (for review see Spence and Parise, 2010) as well as visual eccentricity (Westheimer, 1983). Numerous studies have demonstrated that patients with neglect and/or extinction exhibit pathologically biased temporal order judgments (Baylis et al., 2002; Berberovic et al., 2004; Dukewich et al; 2012; Robertson et al., 1998; Rorden et al., 1997; Rorden et al., 2009; Sinnett et al., 2007) where the item on the contralesional side must be presented much earlier (typically in the order of 200ms) than the item on the ipsilesional side in order to be perceived as being simultaneously. On the other hand, neurologically healthy individuals who are accustomed to left-to-right reading tend to a subtle effect in the opposite direction, tending to perceive the left item as occurring first when confronted with two simultaneous stimuli (for review, see Pérez et al., 2011).
We hypothesized that the pathological temporal order judgment biases observed in stroke patients would be more severe when stimuli were presented on the contralesional side of the individual’s trunk when compared to identical stimuli presented on their trunks’s ipsilesional side. This would provide clear evidence that the temporal deficits observed in the temporal order task interact with or are driven by the spatial biases. Crucially, across all conditions we presented the stimuli at the same locations with respect to the fovea (as eccentricity can influence TOJs, Westheimer, 1983) and the head, (thus varying only trunk-centered egocentric coordinates). We predicted that trunk-based modulation of TOJ would be specific to individuals who actively exhibit the core symptoms of spatial neglect, which are associated with biased egocentric, body-related internal maps (Karnath et al., 1991, 1993; Karnath and Rorden, 2012). To test this hypothesis, we recruited both neurologically healthy controls as well as three groups of chronic stroke survivors: those who never exhibited neglect, those who had exhibited neglect at the acute stage but had recovered by the time of experimental testing and those who still suffered from spatial neglect. We predicted that only the final group had a trunk-based bias and thus would exhibit an interaction between trunk position and perception.
Materials and Methods
Participants
Fifteen consecutively admitted patients with first ever right hemisphere stroke participated in this as well as in a previously reported study (Li et al., 2017). Patients with a left-sided stroke, patients with diffuse or bilateral brain lesions, as well as patients who were unable to follow the instructions to finish the experiment were excluded. All of the patients conducted the initial clinical testing on average 5.1 days post-stroke (SD 4.5) and the second clinical testing in the chronic phase on average 1042.1 days (SD 415.1) post-stroke. We defined neglect as a binomial symptom, based on performance on tasks described below. By this definition, five individuals showed spatial neglect (NEG) in both acute and chronic phase, five of them showed spatial neglect in the acute phase but no longer in the chronic phase (neglect recovered, NR) and the other five did not show spatial neglect at either the acute nor the chronic phase (right brain damaged controls, RBD). Two of the five neglect patients also exhibited chronic visual extinction as assessed by missing at least 50% of contralesional items on bilateral trials (cf. Table 1) using computerized testing (described below). Additionally, fifteen age-matched healthy participants (non-brain damaged controls, NBD) without neurological or psychiatric disorders were tested. All thirty subjects gave their informed consent to participate in the study, which was performed in accordance with the ethical standard of the 1964 Declaration of Helsinki. Demographic and clinical data of all subjects are presented in Table 1.
Table 1.
Demographic and clinical data of all 30 participants.
| NEG | NR | RBD | NBD | |
|---|---|---|---|---|
| Number | 5 | 5 | 5 | 15 |
| Sex(m/f) | 3/2 | 3/2 | 3/2 | 5/10 |
| Age(years) | 73.4(1.52) | 68.6(7.3) | 69(7.55) | 70(4.42) |
| Etiology | 5 Infarct | 3 Infarct | 4 Infarct | |
| 2 Hemorrhage | 1 Hemorrhage | |||
| Lesion size (cc) | 70.26 (70.51) | 42.30 (28.15) | 27.50 (14.32) | |
| Time between lesion and TOJ experiment (days) | 1140.6(461.4) | 1169.6(527.4) | 816(124.8) | |
| Visual field defects (% present) | 0% | 0% | 0% | |
| Visual extinction (% fail to report contralesional stimuli in bilateral displays) | NEG01 75% | 0% | 0% | |
| NEG02 100% | ||||
| NEG03 20% | ||||
| NEG04,05 0% | ||||
| Spatial neglect scores | ||||
| Letter cancellation (CoC) | Acute: 0.51(0.24) | Acute: 0.42(0.25) | Acute: 0.004(0.01) | |
| Chronic: 0.07(0.04) | Chronic: 0 (0.02) | Chronic: 0 (0.01) | ||
|
|
||||
| Bells test (CoC) | Acute: 0.58(0.29) | Acute: 0.26(0.14) | Acute: −0.01(0.02) | |
| Chronic: 0.17(0.16) | Chronic: 0.04(0.04) | Chronic: 0 (0) | ||
|
|
||||
| Copying (% omitted) | Acute: 62.5(17.7) | Acute: 47.5(24.0) | Acute: 0 (0) | |
| Chronic: 12.5(10.2) | Chronic: 0 (0) | Chronic: 0 (0) | ||
Data are presented as mean (standard deviation). CoC, Center of Cancellation (Rorden & Karnath, 2010); NEG, right brain damage with spatial neglect in both acute and chronic phases of stroke; NR, right brain damage with acute spatial neglect but no chronic neglect; RBD, right brain damage without spatial neglect; NBD, non-brain damage; m, male; f, female.
Clinical assessment
All fifteen brain damaged patients were assessed in the acute and in the chronic phase of the stroke with the following clinical neglect tests: Letter Cancellation Task (Weintraub and Mesulam, 1985), Bells Test (Gauthier et al, 1989), and a Copying Task (Johannsen and Karnath. 2004). All three tests were presented on a horizontally oriented 21*29.7cm sheet of paper. For the Letter Cancellation Task and the Bells Test, we calculated the Center of Cancellation (CoC) using the procedure and software by Rorden and Karnath (2010). This measure is sensitive to both the number of omissions and the location of these omissions. CoC scores > 0.09 in the Letter Cancellation Task and the Bells Test were taken to indicate neglect behavior (cf. Rorden and Karnath, 2010). In the Copying Task, omission of at least one of the contralateral features of each figure was scored as 1, and omission of each whole figure was scored as 2. One additional point was given when contralesional figures were drawn on the ipsilesional side of the test sheet. The maximum score was 8. A score higher than 1 (i.e., >12.5% omissions) indicated spatial neglect (Johannsen & Karnath, 2004). For a firm diagnosis of spatial neglect in the acute phase of the stroke, i.e. when the pathological behavior is most extreme, the patients had to fulfill the above criteria in at least two of the three tests. At the time of the second (chronic) assessment, patients were classified as showing chronic neglect when they fulfilled the above criteria in at least one of the three tests.
Visual field defects were examined using the common neurological confrontation technique. Visual extinction was examined using a computerized task. This task included four geometrical figures (square, circle, triangle, diamond), each 0.7° in size, presented for 180ms in random order 4° left and/or right of a central fixation point presented on a PC monitor; stimuli were generated and presented by software E-Prime 1.0 and displayed on a ThinkPad laptop (type 8932) with a screen size of 1280*800 pixel. There were 10 trials with bilateral and 20 trials with unilateral left or right presentations. With this setup, all tested individuals reported at least 90% of the unilateral contralesional and unilateral ipsilesional stimuli. We used the proportion of contralesional stimuli during bilateral stimulation as our measure of visual extinction.
Stimuli and procedure
The TOJ task used stimuli that were generated by using custom PsycToolbox (Kleiner et al., 2007) scripts using Matlab R2013a software and were displayed on a Macbook Pro laptop with a screen size of 1280*800 pixels. The viewing distance was 60 cm and fixation was positioned at the center of the monitor and at eye height. This test was acquired in about an hour, with breaks provided at the participant’s request. All stimuli (letters and fixation) appeared in white (90% brightness) on a uniform gray background (50% brightness). Stimuli were the upper case letter from the alphabet ‘A’ to ‘X’ without the wide letters ‘W’ and ‘M’ (‘Y’ and ‘Z’ were excluded as they are transposed in German versus English keyboards); they were located about 6.8° to the left and right of a central fixation cross. The size of the letters was 1.55° in height and of the central cross 0.2° in height. The fixation cross was always visible. The single experimenter sat behind the computer display, entering responses into an attached external keyboard – this location allowed the experimenter to gauge fixation, head orientation, and eye movements.
The TOJ task was comparable to the experimental design by Rorden et al. (1997) and di Pellegrino et al. (1997; 1998). The experimenter initiated each trial by pressing a keyboard spacebar confirming the participant had achieved fixation. The first letter appeared 500 ms after this keypress. Two different letters were then presented, one to the left and one to the right of fixation, at the same eccentricity. The stimulus-onset-asynchrony (SOA) between the two different letters could be 0, ±83, ±167, ± 250, ±333, ±417, ±500, or ±583 ms and was randomly varied across trials (Figure 1). The participants were asked to verbally report which letter appeared first on each trial. Both letters remained visible until the response was made and recorded by the experimenter, at which point they were replaced by a screen that only displayed the fixation cross. The experimenter also recorded if an eye movement had been detected, and these trials were excluded from further analysis.
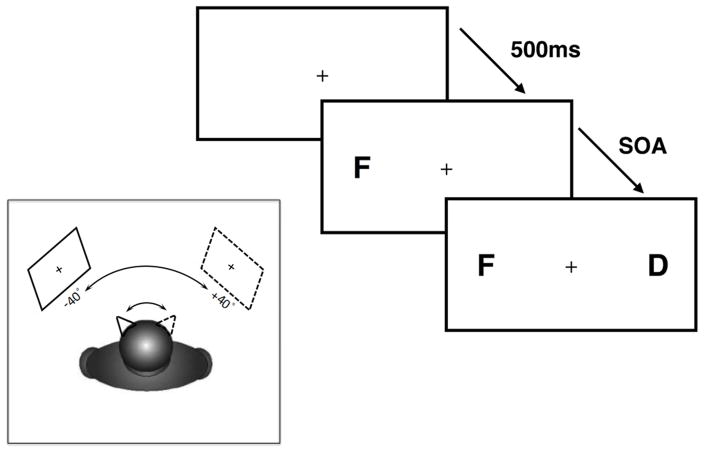
Figure 1.
The main panel shows the time course for each trial: a letter appeared on one side of the display, and following a variable delay a second letter was presented at the opposite location. The task was to simply report the identity of the first letter. The inset cartoon shows the primary manipulation of trunk position: the horizontal position of the center of the presentation monitor was positioned in pseudo-random order either −40° left or +40° right of the subject’s mid-sagittal trunk position at eye level. The subjects were requested to orient head midline and gaze towards the fixation cross at the respective egocentric position while keeping trunk position stable. The retinotopic and the head-centered coordinates of stimulus presentation thus was kept constant throughout the whole experiment; only its position relative to the subject’s trunk was manipulated. We predicted that the temporal deficits measured by the TOJ task would be more severe when the stimuli were presented to the left side of the subject’s mid-sagittal trunk position.
In all fifteen brain damaged patients, the experiment was conducted in the chronic phase of their stroke. There were 9 blocks of stimulus presentation in the whole experiment for each participant with each block containing 4 trials at each of the 15 SOAs (0, ±83, ±167, ± 250, ±333, ±417, ± 500, ±583) in a pseudo-random order (sampled without replacement). The first block served as a training block and was conducted with the center of the presentation monitor aligned with the subject’s straight ahead head and trunk midline at eye level; data were not considered for later analysis. In the following eight experimental blocks, the horizontal position of the center of the presentation monitor was positioned in pseudo-random order (counter balanced between participants) either −40° left or +40° right of the subject’s mid-sagittal trunk position at eye level. The subjects were requested to orient head midline and gaze towards the fixation cross at the respective egocentric position while keeping trunk position stable. The retinotopic and the head-centered coordinates of stimulus presentation (about 6.8° to the left and right of a central fixation cross) thus was kept constant throughout the whole experiment; only its position relative to the subject’s trunk was manipulated. In total, 4 blocks were performed at each egocentric position (for a total of 16 trials at each SOA for each the −40° and 40° egocentric position). Maintaining of gaze and head position was controlled by the experimenter situated opposite of the participating subject. We acknowledge with this design the position of the stimuli relative to objects in the wider environment was altered. However, the workspace of the computer screen (extending well beyond foveal vision: 26.9° horizontally and 17° vertically) and the experimenter (who shifted position to be directly inline with the participants position and the screen’s central fixation cross) were kept constant.
Results
Psignifit (https://github.com/wichmann-lab/psignifit) was used to fit a cumulative Gaussian functions for each condition (trunk orientation) for each patient (Wichmann & Hill, 2001). This allowed us to determine the threshold where the participant was equally likely to report the left as the right item appearing first (the PSS). We also used this fit to estimate a measure of the participant’s variability, we defined this ‘just noticeable difference’ (JND) as the time difference between the fitted function estimating a 37.5% chance of saying ‘right first’ versus the estimation for a 62.5% chance of reporting ‘right first’. These PSS and JND values were used as the dependent measures for our subsequent analyses.
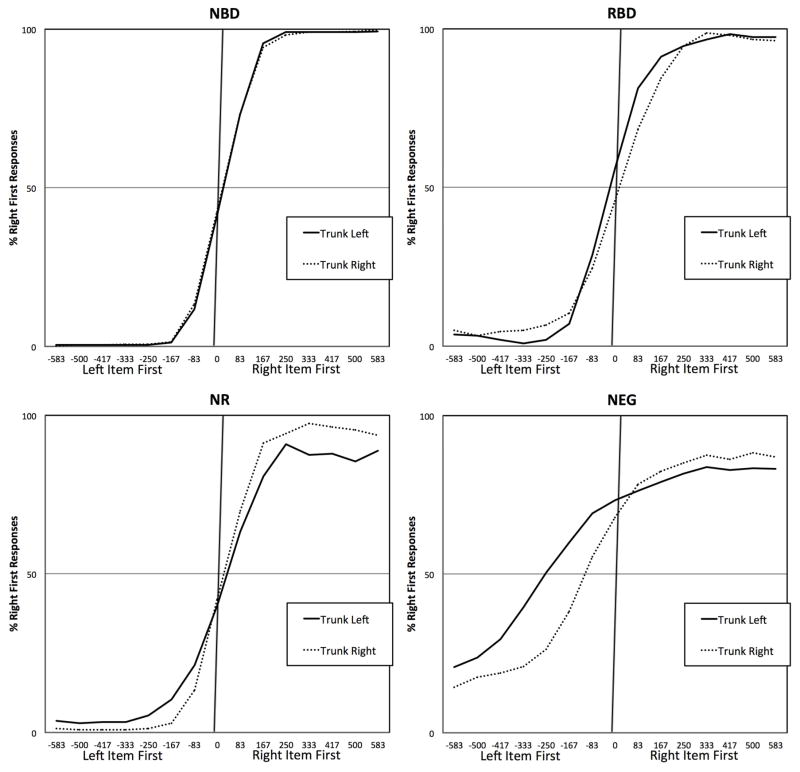
While the fitting was applied to the raw data, we did apply a smoothing function to generate the plots shown in Figure 2. We used the smoothing function described by Rorden et al. (1997) for displaying data which defines the data point for a particular SOA as the average for that SOA plus those immediately on either side of it; each SOA is weighted by its total number of observations when deriving these smoothed averages, smoothing is done on each individuals’ data and the graph illustrates the group mean for this smoothed data. Left-first SOAs are depicted as negative; right-first SOAs as positive. Data did not reveal marked differences in performance depending on whether or not the subjects had additional visual extinction; we thus decided to group the data of all neglect patients. Figure 2 illustrates the percentage of ‘right-then-left’ responses of the four groups for each SOA and each trunk position.
Figure 2.
Mean interpolated results for the four groups tested: no brain damage (NBD), right brain damage with no acute or chronic neglect (RBD), recovered neglect (NR) and chronic neglect (NEG). The vertical axis shows the frequency with which individuals reported the right item appeared first. The horizontal axis shows the stimulus onset asynchrony (in ms), for example ‘−500’ indicates that the left letter appeared one half second before the right letter. A perfect responder would report the right item for 0% of the negative values and 100% of the positive values. Note that as a group, the 15 healthy controls had a small but statistically reliable tendency to report the left item appeared first in the ambiguous case of simultaneous stimulation (an effect previous reported for individuals used to reading left-to-right scripts). On the other hand, the neglect group showed a pathological bias such that the left item needed a substantial lead to be reported as appearing first. Note that this effect is modulated by torso position: with a reduced bias when the display was to the right of the midsagittal midline.
Below we report all results as uncorrected two-tailed p-values. The ANOVA is inherently two-tailed. We have marked tests where we had a specific one-tailed hypothesis with an asterisk. All of these effects were in the hypothesized direction. For contrasts of trunk orientation, we use the term ‘trunk left’ to refer to situations where the stimuli were presented on the left side of the trunk, whereas we use the term ‘trunk right’ to refer to stimuli presented on the right side of the trunk.
We conducted a mixed-design Analysis of Variance (ANOVA) with one between-subject factor (4 levels: healthy controls, NEG, NR, RBD) and one with-in subject factor (two levels: trunk left or trunk right). We used the PSS as the dependent variable. We found a main effect of group (F(3, 26) = 34.772, p < 0.001, η2p = 0.80), as well as an effect for trunk orientation (F(1, 26) = 51.517, p < 0.001, η2p = 0.665) and interaction (F(3, 26) = 34.657, p < 0.001, η2p = 0.80). We conducted an identical ANOVA on the JND data. As four groups can make the interaction hard to decipher, we also conducted a similar ANOVA where the group factor had only two levels: the NEG group and one other. When compared to the NR group we found significant main effects of group (F(1, 8) = 29.093, p = 0.01, η2p = 0.784), trunk orientation (F(1, 8) = 30.198, p = 0.001, η2p = 0.791) and interaction (F(1, 8) = 26.971, p = 0.001, η2p = 0.771). When compared to the RBD group we found significant main effects of group (F(1, 8) = 21.685, p = 0.002, η2p = 0.730), trunk orientation (F(1, 8) = 28.033, p = 0.001, η2p = 0.778) and interaction (F(1, 8) = 17.619, p = 0.003, η2p = 0.688). Relative to the NBD group the analyses revealed found significant main effects of group (F(1, 18) = 83.347, p < 0.001, η2p = 0.822), trunk orientation (F(1, 18) = 95.523, p < 0.001, η2p = 0.841) and interaction (F(1, 18) = 94.925, p < 0.001, η2p = 0.841).
To understand the interaction effect for PSS, we conducted a series of repeated-measures t-tests. For the neglect group, we found a significant difference for trunk orientation (t(4) = 5.54 p = 0.005, CohensD = 2.334) with a more strongly biased PSS for trunk left (mean −282ms, SD 55) versus trunk right (mean −96ms, SD 113). This pattern of ameliorated bias for trunk right was consistent across all participants with trunk left PSS of −286, −188, −326, −290 and −320ms (for patients NEG01 to NEG05 respectively) versus trunk right of −191, 84, −84, −92, −195ms. One-sample t-tests revealed that the PSS significantly deviated from zero in the trunk left (t(4) = −11.372, p < 0.001), but the trunk right orientation did not reach significance (t(4) = −1.890, p = 0.132).
There was no evidence that trunk rotation changed PSS in the NR (t(4) = 0.57, p = 0.596, CohensD = 0.229) or RBD (t(4) = 1.06, p = 0.347, CohensD = 0.544) groups. Likewise, the one-sample t-test did not detect significant deviations from a 0ms bias (NR trunk left: t(4) = 0.81, p = 0.464, mean 11.7ms, SD 32; NR trunk right: t(4) = 0.81, p = 0.464, mean 17ms, SD 16; RBD trunk left: t(4) = 2.32, p = 0.081, mean −13.5ms, SD 18; RBD trunk right: t(4) = 0.3, p = 0.779, mean 8ms, SD 60). We found no evidence that changing trunk position influences PSS in healthy adults (t(14) = 0.10, p = 0.925). Roberts et al. (2012) reported that neurologically healthy adults tend to show a subtle bias in the TOJ task in the opposite direction as neglect patients, e.g. when two stimuli occur objectively simultaneous they tend to report the right item as appearing first, presumably an analog for the pseudo-neglect effects previously reported in healthy individuals on spatial tasks (see Jewell & McCourt, 2000). We observed this predicted effect in single sample t-tests for both the left (t(14) = 3.207, p = 0.006, mean 20.6ms, SD 25) and right (t(14) = 2.875, p = 0.012, mean 20.9ms, SD 28) trunk orientations. For completeness, the PSS (in ms) for all of these individuals for each condition was NR trunk left (−14, −25, 11, 54, 33), NR trunk right (16, −11, 23, 31, 26), RBD trunk left (−22, 1, 2, −7, −42), RBD trunk right (−22, 7, 99, 19, −63), NBD trunk left (19, 17, 30, 41, 10, 8, 48, 87, −18, 10, −8, 5, 19, 28, 13), and NBD trunk right (2, 26, 31, 42, 9, 16, 71, 80, −5, 0, −20, 9, 15, 42, −5).
We conducted an analogous ANOVA using JND as the dependent variable. We found a main effect of group (F(3, 26) = 11.834, p < 0.001, η2p = 0.577), but no effect for trunk orientation (F(1, 26) = 0.070, p = 0.794, η2p = 0.003) or interaction (F(3, 26) = 0.385, p = 0.765, η2p = 0.043). Trunk position did not modulate the JND in any groups (NEG t(4) = 0.21, p = 0.85, 147(SD=112) vs 161(129)ms; NR t(4) = 2.09, p = 0.10, 67(33) vs 42(25)ms; RBD t(4) = 1.46, p = 0.219, 51(17) vs 47(18)ms; NBD t(14) = 0.99, p = 0.34, 32(11) vs 34(10) ms). The main effect of group found in the omnibus ANOVA is revealed by subsequent ANOVAs that compared the neglect group to one group at a time. A trend was observed in the NR group (F(1, 8) = 5.197, p = 0.052, η2p = 0.394), and significant differences for the RBD (F(1, 8) = 6.041, p = 0.039, η2p = 0.430) and NBD (F(1, 18) = 26.680, p < 0.001, η2p = 0.597) groups. In all cases, the direction of these effects was for the NEG group to have more variable responses (mean JND 154ms) versus the other groups (NR=54ms, RBD=49ms, NBD=33ms).
Discussion
Consistent with most prior studies, we observed that individuals with spatial deficits following right hemisphere injury tend to report the right item as occurring first unless the left item has a substantial temporal lead (Baylis et al., 2002; Berberovic et al., 2004; Dukewich et al; 2012; Roberts et al., 2012; Robertson et al., 1998; Rorden et al., 1997; Rorden et al., 2009; Sinnett et al., 2007, Van der Stigchel and Nijboer, 2017). The novel discovery of this work is that this effect tends to be modulated by the position of the stimuli relative to the patient’s trunk. Specifically, participants with chronic neglect exhibit a much more biased PSS (mean for NEG was −282ms) for stimuli presented on the left side of the trunk rather than identical stimuli presented on the right side of the trunk (−95ms). This finding suggests that the temporal biases associated with visual stimuli in spatial neglect are modulated by their position relative to the subject’s trunk.
We note that a couple of cases have been reported where individuals with chronic attentional deficits did not exhibit the expected TOJ bias: one individual described by Dove et al. (2007) and one of the three participants where PSS was reported by Dukewich et al. (2012). We speculate that these individuals may be similar to our ‘neglect recovered’ group, who had very mild symptoms on conventional tasks and as a group did not exhibit reliable PSS biases. It is possible that during rehabilitation many individuals learn to compensate for pathological attentional biases by adapting their top down control to attend to the weak side. Such top-down strategies have been shown to influence PSS in healthy adults (Stelmach and Herdman, 1991). We further suggest that unspeeded tasks such as the TOJ task employed here may not be as sensitive as reaction time tasks to subtle chronic deficits of attention and cuing (Bonato and Deouell, 2013; Dukewich et al., 2012; Rengachary et al., 2009). In sum, we do not espouse the TOJ task as an ideal measure for chronic spatial biases, rather our goal was to demonstrate that the pathological temporal biases observed in this task can be modulated by simply realigning trunk orientation. Regardless, in our own sample we observed that each individual with chronic neglect exhibited a numerically biased PSS which was ameliorated when the task was in ipsilesonal space with respect to the torso.
The data presented here parallels our recent finding that the pathological attentional blink associated with spatial neglect is modulated by trunk position (Li et al., 2017). Healthy individuals tend to exhibit a brief ‘attentional blink’, where people fail to detect the presence of a target if it comes shortly after a target that they were required to identify. On the other hand, individuals with spatial neglect show a pathologically long attentional blink (Shapiro et al., 2017) which might indicate non-spatial perceptual deficit. Furthermore, the present data is consistent with our companion studies suggesting that trunk position can influence the object-based deficits seen in neglect (Karnath et al., 2011; Li et al., 2014). Individuals with spatial neglect can ignore both the left side of the observers trunk (egocentric deficits) as well as the left side within individual objects (allocentric deficits). For example, when asked to copy a picture of a forest, an individual might fail to reproduce any items on the contralesional side of the picture (egocentric deficit) as well as failing to add details to items on the contralesional side of individual trees. Some suggest that these symptoms dissociate both in behavior and anatomy (e.g. some patients have pure egocentric deficits, while others have pure allocentric deficits; Marsh and Hillis, 2008), whereas others argue that these symptoms associate (with differences between allocentric and egocentric biases reflecting strategic decisions by the patient; Karnath & Niemeier, 2002; Baylis et al., 2004). We observed that neglect of an object’s left side was more severe at contralesional egocentric, trunk-centered positions and ameliorated continuously towards more ipsilesional egocentric positions (Karnath et al., 2011; Li et al., 2014).
In sum, we found that the temporal biases were tightly correlated with the spatial biases. Specifically, the patients identified as having chronic neglect (based on a series of spatial tasks) at the time point when the present experiment was carried out consistently exhibited temporal biases, whereas these temporal biases were not observed in individuals from the three groups (NR, RBD, NBD) who did not exhibit spatial biases. This association seems to suggest the temporal biases and spatial biases do not rely on independent modules. Indeed, several studies have noted that biases in the TOJ task correlate with spatial neglect as measured by cancellation tasks though not with extinction (Roberts et al., 2012) or line bisection deficits (Van der Stigchel and Nijboer, 2017). This could suggest that TOJ deficits correlate with the core deficits of spatial neglect (Karnath & Rorden, 2012), whereas line bisection (Rorden et al., 2006) and extinction (Karnath et al., 2003) are associated with different anatomical injuries. We concede that it is logically possible that there is a ‘when’ module that is independent from a neighboring ‘spatial neglect’ region, with the close spatial proximity meaning that they tend to be injured together. However, we assert that a more parsimonious explanation is that a common impairment biases performance on both the temporal and spatial tasks. While there is considerable evidence that brain regions between the ‘where’ and ‘what’ system are implicated in timing tasks (Battelli et al., 2007; Battelli et al., 2008) we contend that the function of this area is not limited to temporal tasks, and therefore the ‘when’ system term may be misleading. We suggest that this brain area may have a more general role in salience marking (Corbetta and Shulman, 2002). On the other hand, we note that Roberts et al. (2012) associated the biased PSS in TOJ tasks with both damage to the temporoparietal cortex as well as classic neglect symptoms, whereas they associated generally poor temporal resolution of TOJs with damage to the right parietal lobe. This suggests that the TOJ task can detect two dissociable deficits, a spatial bias due to neglect as well as a temporal degradation associated with disruption of the putative “when” system. While the present work provides strong support for the former, the present work can not refute the latter possibility. Regardless, the current finding emphasizes the interaction of egocentric spatial position on the temporal order perception biases observed following stroke.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/23-1) and the National Institutes of Health (P50DC014664). We are grateful to Daniel Wiesen for his help with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Battelli L, Pascual-Leone A, Cavanagh P. The “when” pathway of the right parietal lobe. Trends Cogn Sci. 2007;11:204–210. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L, Walsh V, Pascual-Leone A, Cavanagh P. The ‘when’ parietal pathway explored by lesion studies. Curr Opin Neurobiol. 2008;18(2):120–6. doi: 10.1016/j.conb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Simon SL, Baylis LL, Rorden C. Visual extinction with double simultaneous stimulation: what is simultaneous? Neuropsychologia. 2002;40(7):1027–34. doi: 10.1016/s0028-3932(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Baylis LL, Gore CL. Visual neglect can be object based or scene-based depending on task representation. Cortex. 2004;40:237–246. doi: 10.1016/s0010-9452(08)70119-9. [DOI] [PubMed] [Google Scholar]
- Becchio C, Bertone C. Time and neglect: abnormal temporal dynamics in unilateral spatial neglect. Neuropsychologia. 2006;44(14):2775–82. doi: 10.1016/j.neuropsychologia.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Berberovic N, Pisella L, Morris AP, Mattingley JB. Prismatic adaptation reduces biased temporal order judgements in spatial neglect. Neuroreport. 2004;15(7):1199–204. doi: 10.1097/00001756-200405190-00024. [DOI] [PubMed] [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP. Distinct syndromes of hemineglect. Arch Neurol. 1992;49(11):1187–94. doi: 10.1001/archneur.1992.00530350109026. [DOI] [PubMed] [Google Scholar]
- Bonato M, Deouell LY. Hemispatial neglect: computer-based testing allows more sensitive quantification of attentional disorders and recovery and might lead to better evaluation of rehabilitation. Front Hum Neurosci. 2013;7:162. doi: 10.3389/fnhum.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Basso G, Frassinetti F. Spatial extinction on double asynchronous stimulation. Neuropsychologia. 1997;35(9):1215–23. doi: 10.1016/s0028-3932(97)00044-4. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Basso G, Frassinetti F. Visual extinction as a spatio-temporal disorder of selective attention. Neuroreport. 1998;9(5):835–9. doi: 10.1097/00001756-199803300-00013. [DOI] [PubMed] [Google Scholar]
- Dove ME, Eskes G, Klein RM, Shore D. A left attentional bias in chronic neglect: a case study using temporal order judgments. Neurocase. 2007;13(1):37–49. doi: 10.1080/13554790601174146. [DOI] [PubMed] [Google Scholar]
- Driver J, Pouget A. Object-centered visual neglect, or relative egocentric neglect? J Cogn Neurosci. 2000;12:542–545. doi: 10.1162/089892900562192. [DOI] [PubMed] [Google Scholar]
- Dukewich KR, Eskes GA, Lawrence MA, Macisaac MB, Phillips SJ, Klein RM. Speed impairs attending on the left: comparing attentional asymmetries for neglect patients in speeded and unspeeded cueing tasks. Front Hum Neurosci. 2012;6:232. doi: 10.3389/fnhum.2012.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38(1):93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Schenkel P, Fischer B. Trunk orientation as the determining factor of ‘contralateral’ deficit in the neglect syndrome and as the physical anchor of the internal representation of body orientation in space. Brain. 1991;114:1997–2014. doi: 10.1093/brain/114.4.1997. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Christ K, Hartje W. Decrease of contralateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain. 1993;116:383–396. doi: 10.1093/brain/116.2.383. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Küker W. The cortical substrate of visual extinction. Neuroreport. 2003;14(3):437–42. doi: 10.1097/01.wnr.0000059778.23521.88. Erratum 14: 1189. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Niemeier M. Task-dependent differences in the exploratory behaviour of patients with spatial neglect. Neuropsychologia. 2002;40:1577–1585. doi: 10.1016/s0028-3932(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Mandler A, Clavagnier S. Object-based neglect varies with egocentric position. Journal of Cognitive Neuroscience. 2011;23:2983–2993. doi: 10.1162/jocn_a_00005. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Rorden C. The anatomy of spatial neglect. Neuropsychologia. 2012;50(6):1010–1017. doi: 10.1016/j.neuropsychologia.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO. Spatial attention systems in spatial neglect. Neuropsychologia. 2015;75:61–73. doi: 10.1016/j.neuropsychologia.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D. Perception 36 ECVP Abstract Supplement. 2007. What’s new in Psychtoolbox-3? [Google Scholar]
- Li D, Karnath H-O, Rorden C. Egocentric representations of space co-exist with allocentric representations: evidence from spatial neglect. Cortex. 2014;58:161–169. doi: 10.1016/j.cortex.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Rorden C, Karnath HO. “Nonspatial” Attentional Deficits Interact with Spatial Position in Neglect. J Cogn Neurosci. 2017;29(5):911–918. doi: 10.1162/jocn_a_01101. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE. Dissociation between egocentric and allocentric visuospatial and tactile neglect in acute stroke. Cortex. 2008;44(9):1215–20. doi: 10.1016/j.cortex.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke K, Kagan I, Wüstenberg T, Bähr M, Wilke M. Trunk rotation affects temporal order judgments with direct saccades: Influence of handedness. Neuropsychologia. 2015;79:123–37. doi: 10.1016/j.neuropsychologia.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Pérez A, García L, Valdés-Sosa M, Jaśkowski P. Influence of the Learnt Direction of Reading on Temporal Order Judgments. Psychology. 2011;2(2):103–108. [Google Scholar]
- Priftis K, Bonato M, Zorzi M, Umiltà C. Spatial and non-spatial aspects of neglect. Front Hum Neurosci. 2013;7:25. doi: 10.3389/fnhum.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, d’Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Arch Phys Med Rehabil. 2009;90(12):2081–8. doi: 10.1016/j.apmr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Lau JK, Chechlacz M, Humphreys GW. Spatial and temporal attention deficits following brain injury: a neuroanatomical decomposition of the temporal order judgement task. Cogn Neuropsychol. 2012;29(4):300–24. doi: 10.1080/02643294.2012.722548. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395(6698):169–72. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Rorden C, Mattingley JB, Karnath HO, Driver J. Visual extinction and prior entry: impaired perception of temporal order with intact motion perception after unilateral parietal damage. Neuropsychologia. 1997;35(4):421–33. doi: 10.1016/s0028-3932(96)00093-0. [DOI] [PubMed] [Google Scholar]
- Rorden C, Fruhmann Berger M, Karnath H. Disturbed line bisection is associated with posterior brain lesions. Brain Res. 2006;1080:17–25. doi: 10.1016/j.brainres.2004.10.071. [DOI] [PubMed] [Google Scholar]
- Rorden C, Jelsone L, Simon-Dack S, Baylis LL, Baylis GC. Visual extinction: the effect of temporal and spatial bias. Neuropsychologia. 2009;47(2):321–9. doi: 10.1016/j.neuropsychologia.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett S, Juncadella M, Rafal R, Azañón E, Soto-Faraco S. A dissociation between visual and auditory hemi-inattention: Evidence from temporal order judgements. Neuropsychologia. 2007;45(3):552–60. doi: 10.1016/j.neuropsychologia.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body- and world-referenced representations of visual space in parietal cortex. Nature. 1998;394(6696):887–91. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- Spence C, Parise C. Prior-entry: a review. Conscious Cogn. 2010;19(1):364–79. doi: 10.1016/j.concog.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Stelmach LB, Herdman CM. Directed attention and perception of temporal order. J Exp Psychol Hum Percept Perform. 1991;17(2):539–50. doi: 10.1037//0096-1523.17.2.539. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Nijboer TC. Temporal order judgements as a sensitive measure of the spatial bias in patients with visuospatial neglect. J Neuropsychol. 2017 doi: 10.1111/jnp.12118. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Temporal order detection for foveal and peripheral visual stimuli. Vision Res. 1983;23:759–763. doi: 10.1016/0042-6989(83)90197-9. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 2001;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]