Abstract
Introduction
In a meta-analysis, we observed a significant 37% relative risk reduction in prospectively adjudicated major adverse cardiac events [MACEs, comprising of non-fatal myocardial infarction, non-fatal stroke, cardiovascular (CV) death] with vildagliptin vs. comparators in younger (< 65 years) patients with type 2 diabetes mellitus (T2DM), while the risk was similar in older patients (≥ 65 years). We carried out an exploratory analysis to identify the patient characteristics and on-treatment effects that may have contributed to the different outcomes in the two age groups.
Methods
On-treatment differences (vildagliptin vs. comparators) for the change from baseline in CV risk factors were analyzed using an analysis of covariance model with the baseline value for each variable of interest, treatment and study as covariates. Additional adjustments for background antihypertensive and statin use were performed when analyzing changes in blood pressure and lipids, respectively. Baseline characteristics and patient demographics were analyzed using descriptive statistics.
Results
Patients aged < 65 years had shorter diabetes duration (4.4 vs. 8.2 years) and slightly higher glycated hemoglobin (HbA1c) at baseline (8.3% vs. 8.0%) than patients aged ≥ 65 years. More patients in the ≥ 65 year age group had hypertension (73.1% vs. 51.3%), dyslipidemia (53.3% vs. 43.9%) and a history of CV events (32.2% vs. 12.9%). There were small, but statistically significant differences in the change in HbA1c and total cholesterol in favor of vildagliptin relative to comparators, which were similar in both age groups. Significant differences were observed in the reduction in systolic blood pressure (SBP) (− 0.52 mmHg; 95% CI − 0.97, − 0.07; p = 0.023), low-density lipoprotein (LDL cholesterol) (− 0.12 mmol/l; 95% CI − 0.19, − 0.04; p = 0.002) and weight (− 0.48 kg; 95% CI − 0.95, − 0.01; p < 0.047) in patients < 65 years, but not in patients ≥ 65 years. The incidence of hypoglycemic events was lower in patients treated with vildagliptin [2.1 and 3.5 per 100 subject years exposure (SYEs) in < 65 and ≥ 65 years, respectively] than with comparators (5.8 and 7.5 per 100 SYEs, respectively).
Conclusion
Based on our findings, it can be hypothesized that the positive effects of vildagliptin on SBP, LDL cholesterol, hypoglycemia and weight observed in younger, but not in older patients could be associated with the lower risk of MACE in younger patients with T2DM.
Funding
Novartis.
Keywords: Cardiovascular risk, DPP-4 inhibitor, Type 2 diabetes mellitus, Vildagliptin
Introduction
Type 2 diabetes mellitus (T2DM) is an independent risk factor for developing cardiovascular (CV) disease, with an estimation that every percentage increase in glycated hemoglobin (HbA1c) increases the relative risk of CV disease by 18% [1, 2]. A prior CV event in patients with T2DM increases CV morbidity and mortality, and this occurs at an earlier age and at a higher rate than in the non-diabetic population [3, 4]. In addition, CV safety signals with some antidiabetic agents over the past decade prompted the regulatory bodies to mandate the demonstration of CV safety of a new agent before its approval. As per health authority guidance, CV safety of a drug is considered confirmed if a CV meta-analysis of clinical trials or a CV outcome study shows that the upper limit of the 95% confidence interval (CI) for the risk ratio drug vs. comparators for important CV events is < 1.3 [5]. Moreover, a comprehensive assessment of the CV risk–benefit profile of antidiabetic agents provides relevant clinical evidence to guide adequate management of patients with T2DM and CV disease.
Three large CV outcome trials (SAVOR-TIMI-53, EXAMINE and TECOS) with dipeptidyl peptidase-4 (DPP-4) inhibitors [6–8] did not report an overall increased risk of major adverse CV events (MACE); however, there was a minor imbalance in hospitalizations for heart failure with alogliptin and saxagliptin. In an early prospectively planned meta-analysis of independently adjudicated CV events (acute coronary syndrome, stroke, transient ischemic attack with imaging evidence of infarction or CV death) from phase III trials of the vildagliptin clinical trial program, vildagliptin 50 mg twice daily (bid) did not show increased risk of cardio-cerebrovascular events vs. comparators [upper limit of 95% CI of the Mantel–Haenszel risk ratio (M–H RR) was 1.14] [9].
A more recent meta-analysis of MACE consisting of adjudicated non-fatal myocardial infarction (MI), non-fatal stroke and CV death [10], including data from all the randomized phase III–IV trials of vildagliptin, reaffirmed the CV safety of vildagliptin vs. comparators [M–H RR 0.82 (95% CI 0.61, 1.11)].
In younger patients, diabetes increases the risk of life-years lost because of CV disease, which emphasizes the need for intensified treatment in this increasing population [11, 12]. In the latest meta-analysis, an intriguing finding was observed: the pre-defined subgroup analysis of MACE by age demonstrated a significant 37% reduction in relative risk with vildagliptin in patients < 65 years of age [M–H RR 0.63 (95% CI 0.42, 0.95)], with no increased risk in patients aged ≥ 65 years [M–H RR 1.09 (95% CI 0.70, 1.71)] [10]. In terms of numbers needed to treat (NNT), 227 patients aged < 65 years would need to be treated with vildagliptin over a 1-year period to prevent the occurrence of one MACE. Based on these findings, we carried out an exploratory analysis to identify the plausible differences in the baseline patient characteristics and on-treatment effects that may have contributed to the observed risk reduction with vildagliptin in patients aged < 65 years.
Methods
Study Design and Patients
Patient-level data from 37 monotherapy or combination therapy studies, including oral antidiabetic drugs (OADs) and insulin, comparing vildagliptin [50 mg once daily (qd)/bid] with all comparators, i.e., placebo or an active comparator [sulfonylureas (SUs), metformin, thiazolidinediones (TZDs), etc.], wherein independent adjudication of adverse CV events was performed, were pooled for this analysis. The proportion of the comparators in the study population of the current sub-analysis is similar to that in the primary publication [10]. All randomized phase III–IV studies completed on or before December 2013 were included in this analysis, and key study characteristics were described in the primary publication [10].
Assessments and Statistical Analysis
Patients' demographic data and baseline characteristics were summarized using descriptive statistics for the two age groups by treatment—vildagliptin 50 mg qd/bid or comparator. Standard Medical Dictionary for Regulatory Activities (MedDRA) queries were used to identify and report history of CV events. Changes in HbA1c, fasting plasma glucose (FPG), weight, micro and macro albuminuria, estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides from baseline to endpoint by age group and treatment allocation were analyzed using the analysis of covariance (ANCOVA) model, with the respective baseline, treatment and study as covariates. The incidence of hypoglycemia by treatment and age groups is also summarized descriptively. For the analysis of change in blood pressure (BP), lipids and albuminuria/eGFR covariates also included antihypertensive medication at baseline, statin at baseline and changes in SBP, DBP and HbA1c, respectively.
Ethics and Good Clinical Practice
All studies included in this analysis were conducted in accordance with Good Clinical Practice and in accordance with the Declaration of Helsinki. All study protocols were approved by an independent ethics committee or institutional review board. A written informed consent was collected from all participants to participate in the respective trials included in this pooled analysis.
Results
Study Population and Treatment
Data from 16,701 patients were pooled for the current analysis, 9599 (57.5%) received vildagliptin 50 mg qd/bid and 7102 (42.5%) received comparators. The majority of the patients were aged < 65 years (N = 12,358; 74%), and patients receiving vildagliptin or the comparator were equally distributed across the two age groups: 7239 (58.6%) received vildagliptin and 5119 (41.4%) received the comparator in the age < 65 years group; 2360 (54.3%) received vildagliptin and 1983 (45.7%) received comparator in the age ≥ 65 years group.
Baseline Characteristics and Demographics
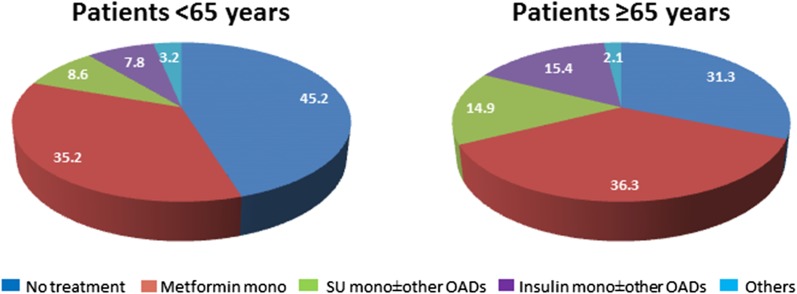
Overall, the baseline characteristics were similar between the vildagliptin and comparator groups when stratified by age. The younger age group was characterized by lower mean diabetes duration (< 5 years), slightly higher HbA1c (8.3% vs. 8.0%) and body weight (86.8 vs. 80.0 kg); less than 20% were treated with SU/insulin alone or in combination with other oral antidiabetic agents (Fig. 1). As expected more patients in the ≥ 65 year age group had CV risk factors such as hypertension, dyslipidemia, moderate or severe renal impairment and a history of CV events. There were more Asians in the < 65 year age group compared with the ≥ 65 year age group (Table 1).
Fig. 1.
Percentage distribution of antidiabetic medication in patients aged < 65 and ≥ 65 years at baseline. SU sulfonylurea, OADs oral antidiabetic drugs
Table 1.
Key demographic and background characteristics of the study population* by age
| Parameters | Age < 65 years | Age ≥ 65 years | ||
|---|---|---|---|---|
| VILDA n = 7239 |
COMP n = 5119 |
VILDA n = 2360 |
COMP n = 1983 |
|
| Age (years) | 52.0 ± 8.3 | 52.4 ± 8.2 | 70.3 ± 4.2 | 70.2 ± 4.2 |
| Male (%) | 4106 (56.7) | 2882 (56.3) | 1204 (51.0) | 1046 (52.7) |
| Diabetes duration (years) | 4.4 ± 5.2 | 4.9 ± 5.5 | 8.2 ± 8.0 | 9.0 ± 8.2 |
| Race | ||||
| Caucasian | 4309 (59.5) | 3035 (59.3) | 1734 (73.5) | 1464 (73.8) |
| Asian | 1752 (24.2) | 1244 (24.3) | 413 (17.5) | 358 (18.1) |
| Hispanic or Latino | 783 (10.8) | 560 (10.9) | 146 (6.2) | 120 (6.1) |
| Black | 302 (4.2) | 193 (3.8) | 41 (1.7) | 25 (1.3) |
| Other | 93 (1.3) | 87 (1.7) | 26 (1.1) | 16 (0.8) |
| Body weight (kg) | 86.8 ± 19.5 | 86.3 ± 19.6 | 80.0 ± 15.8 | 79.9 ± 16.1 |
| BMI (kg/m2) | 30.8 ± 5.7 | 30.7 ± 5.8 | 29.5 ± 4.8 | 29.5 ± 4.9 |
| BMI ≥ 35 | 1712 (23.6) | 1150 (22.5) | 327 (13.9) | 266 (13.4) |
| HbA1c (%) | 8.3 ± 1.1 | 8.2 ± 1.1 | 8.0 ± 1.1 | 7.9 ± 1.0 |
| eGFR (MDRD) (ml/min/1.73 m2)a | ||||
| Moderate (≥ 30–< 50) | 109 (1.5%) | 72 (1.4%) | 282 (11.9%) | 237 (12.0%) |
| Severe (< 30) | 99 (1.4%) | 76 (1.5%) | 121 (5.1%) | 92 (4.6%) |
| Hypertension | 3712 (51.3) | 2808 (54.9) | 1724 (73.1) | 1453 (73.3) |
| Dyslipidemia | 3177 (43.9) | 2237 (43.7) | 1258 (53.3) | 1025 (51.7) |
| History of CV events | 932 (12.9) | 679 (13.3) | 761 (32.2) | 606 (30.6) |
| Patients with age risk factor plus either hypertension or dyslipidemia | 1918 (26.5%) | 1432 (28.0%) | 1984 (84.1%) | 1669 (84.2%) |
| Antihypertensives (overall) | 3321 (45.9%) | 2539 (49.6%) | 1731 (73.3%) | 1456 (73.4%) |
| Lipid modifying agents (overall) | 1961 (27.1%) | 1487 (29.0%) | 1039 (44.0%) | 863 (43.5%) |
| Anti-platelet medications | 1313 (18.1%) | 972 (19.0%) | 814 (34.5%) | 668 (33.7%) |
Data are mean ± SD or n (%) unless otherwise mentioned
* Pooled data from vildagliptin 50 mg qd/bid randomized, controlled double-blind phase III studies
bid twice daily, BMI body mass index, CV cardiovascular, COMP comparators, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, MDRD the modification of diet in renal disease, qd once daily, SD standard deviation, VILDA vildagliptin
aeGFR (MDRD) = GFR estimated using the MDRD formula
On-treatment Differences in Cardio-metabolic Parameters
Adjusted mean changes and placebo-corrected values for various cardio-metabolic parameters including glycemic levels (HbA1c and FPG), weight, lipids (LDL cholesterol, HDL cholesterol, total cholesterol and triglycerides), BP (SBP and DBP) and eGFR are presented in Table 2. There were small, but statistically significant differences in the change in HbA1c, HDL cholesterol and total cholesterol in favor of vildagliptin vs. comparator, which were seen in both age groups (Table 2).
Table 2.
Adjusted mean change in parameters from baseline to endpoint in age-stratified subgroups
| Parameters | Adjusted mean change (SE) | Difference in adjusted mean change (VILDA-COMP) | ||||
|---|---|---|---|---|---|---|
| < 65 years | ≥ 65 years | Mean (SE); 95% CI; p value | ||||
| VILDA n = 7239 |
COMP n = 5119 |
VILDA n = 2360 |
COMP n = 1983 |
< 65 years | ≥ 65 years | |
| HbA1c (%) | − 0.69 (0.02) | − 0.54 (0.02) | − 0.71 (0.02) | − 0.43 (0.03) | − 0.14 (0.02); (− 0.19, − 0.10); p < 0.001* | − 0.28 (0.03); (− 0.34, − 0.22); p < 0.001* |
| Weight (kg) | 0.33 (0.32) | 0.81 (0.33) | 0.78 (0.38) | 0.76 (0.40) | − 0.48 (0.24); (− 0.95, − 0.01); p = 0.047* | 0.02 (0.33); (− 0.63, 0.67); p = 0.949 |
| HDL cholesterol (mmol/l) | 0.02 (0.00) | 0.04 (0.00) | 0.01 (0.01) | 0.02 (0.01) | − 0.02 (0.00); (− 0.03, − 0.01); p < 0.001* | − 0.01 (0.01); (− 0.03, 0.00); p = 0.035* |
| LDL cholesterol (mmol/l) | 0.00 (0.05) | 0.12 (0.05) | − 0.08 (0.05) | − 0.09 (0.06) | − 0.12 (0.04); (− 0.19, − 0.04); p = 0.002* | 0.01 (0.06); (− 0.11, 0.12); p = 0.928 |
| Total cholesterol (mmol/l) | − 0.19 (0.02) | − 0.06 (0.02) | − 0.14 (0.02) | − 0.07 (0.02) | − 0.13 (0.02); (− 0.16, − 0.10); p < 0.001* | − 0.07 (0.03); (− 0.12, − 0.02); p = 0.005* |
| DBP (mmHg) | − 1.59 (0.12) | − 1.46 (0.13) | − 1.71 (0.21) | − 1.76 (0.22) | − 0.13 (0.15); (− 0.42, 0.15); p = 0.359 | 0.05 (0.25); (−0.44, 0.53); p = 0.852 |
| SBP (mmHg) | − 1.91 (0.18) | − 1.39 (0.20) | − 2.55 (0.35) | − 3.05 (0.38) | − 0.52 (0.23); (− 0.97, − 0.07); p = 0.023* | 0.50 (0.42); (− 0.32, 1.32); p = 0.232 |
| Triglycerides (mmol/l) | − 0.14 (0.05) | − 0.13 (0.05) | − 0.12 (0.03) | − 0.11 (0.03) | − 0.01 (0.03); (− 0.06, 0.04); p = 0.706 | − 0.01 (0.03); (− 0.06, 0.04); p = 0.747 |
| FPG (mmol/l) | − 0.86 (0.04) | − 0.81 (0.04) | − 0.94 (0.06) | − 0.71 (0.06) | − 0.06 (0.05); (− 0.15, 0.03); p = 0.214 | − 0.24 (0.07); (− 0.37, − 0.10); p < 0.001* |
| eGFR (MDRD) (ml/min) | − 3.37 (1.13) | − 2.67 (1.28) | − 0.93 (0.72) | − 1.71 (0.73) | − 0.70 (1.60); (− 3.85, 2.45); p = 0.663 | 0.79 (0.94) (− 1.06, 2.63); p = 0.403 |
| Micro-/macro albuminuria (mg/mmol) | 0.99a (0.96, 1.02) | 1.01a (0.97, 1.04) | 0.98a (0.92, 1.04) | 1.02a (0.96, 1.09) | 0.98b (0.95, 1.02); p = 0.363 | 0.96b (0.89, 1.03); p = 0.233 |
COMP comparators, CI confidence intervals, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, FPG fasting blood glucose, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein, MDRD the modification of diet in renal disease, SBP systolic blood pressure, SE standard error of mean, VILDA vildagliptin
* Statistical significance at 5% level
aRatio: endpoint/baseline geometric mean
bRatio: VILDA/COMP
Significant on-treatment changes in favor of vildagliptin were observed for SBP (− 0.52 mmHg; 95% CI − 0.97, − 0.07; p = 0.023), LDL cholesterol (− 0.12 mmol/l; 95% CI − 0.19, − 0.04; p = 0.002) and bodyweight (− 0.48 kg; 95% CI − 0.95, − 0.01; p < 0.05) in the patients < 65 years, which were not observed in the ≥ 65 year age group. The exposure-adjusted incidence of hypoglycemic events was lower in patients treated with vildagliptin (2.1 and 3.5 per 100 subject years of exposure [SYEs] in the < 65 and ≥ 65 year groups, respectively) than with comparators (5.8 and 7.5 per 100 SYEs, respectively).
Discussion
The results from this exploratory analysis show a small favorable effect of vildagliptin on SBP, weight and LDL cholesterol in patients aged < 65 years with a low prevalence of prior CV disease, whereas a similar effect with vildagliptin was observed in HbA1c, HDL cholesterol and total cholesterol in both the younger and older age groups. Whether this favorable effect on cardio-metabolic risk factors might explain the observed relative risk reduction in MACE in the meta-analysis comparing vildagliptin 50 mg qd/bid versus all comparators in phase III and phase IV randomized controlled trials (RCTs) remains to be confirmed. In addition to the significant glucose-lowering effect [13, 14], vildagliptin has been shown to reduce blood pressure and improve fasting lipid profiles in association with reductions in weight [15]. The lower incidence of hypoglycemic events in the younger patients may also have played a role in the reduction of the risk of MACE in this group. A complex interplay of various factors such as hyperglycemia, hypoglycemia, hypertension, body weight and dyslipidemia may increase the risk of CV disease in patients with T2DM [16, 17]. Thus, although the influence of various cardio-metabolic parameters on the observed CV risk reduction with vildagliptin could not be ascertained, we can speculate that the favorable effect on multiple cardio-metabolic effects might be acting synergistically to the benefit of younger T2DM patients with fewer CV risk factors.
The finding of a significant risk reduction in the incidence of MACE with vildagliptin in the subgroup of patients aged < 65 years is intriguing since none of the CV outcome studies with the DPP-4 inhibitors suggested such potential benefit. Although a direct comparison between the results from the vildagliptin meta-analysis and the three large CV outcome trials with DPP-4 inhibitors (SAVOR-TIMI-53, EXAMINE and TECOS [6–8]) is inappropriate, one can draw useful insights by theoretical comparison. In these studies, all patients had established CV disease, which may have prevented achieving a CV benefit similarly to the neutral effect observed in the patients ≥ 65 years in our analysis. This observation is supported by a meta-analysis in an older population (> 65 years) initiating DPP-4 inhibitors vs. other therapeutic alternatives, which showed that there was no increased risk of cardiovascular events in this population, which generally is at risk of CV events [18]. So, in patients with T2DM at high CV risk, an aggressive treatment of risk factors other than hyperglycemia such as hypertension, cholesterol and triglycerides is required to reduce CV events, as shown in the Steno-2 study [19, 20]. Another important difference is that the vildagliptin meta-analysis included data from all comparators, including SUs, while the comparator in the CV outcome studies was placebo. It is suggested that SUs can trigger mechanisms that could compromise CV safety [21], so it could be hypothesized that the vildagliptin meta-analysis might have benefited from the theoretical (so far) increased risk in patients exposed to SUs. To elucidate this, results from two CV outcome trials, CAROLINA [22] and TOSCA IT [23], are awaited in 2018 to provide more definitive answers regarding the effect of SUs on CV risk relative to other antihyperglycemic drugs.
Three recently completed CV outcome trials, the EMPA-REG [24], LEADER [25] and CANVAS program [26], in T2DM patients with established CV disease, demonstrated a comparable 13–14% risk reduction in the 3-point MACE outcome (CV death, non-fatal myocardial infarction or stroke), while a reduction in all-cause mortality was only observed with the addition of the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) empagliflozin and the glucagon-like peptide -1 receptor analog liraglutide. Although further investigations are needed to fully elucidate the mechanisms responsible for improvement in the CV outcomes in these trials, it has been speculated that the observed benefit by SGLT-2i’s may be attributable to observed differences in established risk factors such as BP, weight and changes in vasculature, while that in the case of liraglutide is mainly due to either the prevention of atherosclerotic progression or plaque biology [24–26]. However, the lack of observed CV benefits in the primary prevention cohort of the CANVAS program with canagliflozin, which enrolled only patients with risk factors but no CV disease at baseline [26], indicates how the CV benefits observed in these CVOTs have limited generalizability outside the studied populations. Therefore, the early benefits of glycemic control on CV outcomes remain to be explored in studies separate from these CVOTs driven by regulatory requirements and primarily designed for the demonstration of CV safety of newer antidiabetic agents in high-risk populations. Demonstrating CV benefit with antidiabetic treatments early in the time course of the disease requires long-term follow-up data in thousands of patients. In the UKPDS, only after initial intensive treatment for a median of 10 years with metformin [27] and an additional 10 years of follow-up, for insulin/SU-based treatment, was a clinically and statistically significant decrease in the risk of MI and all-cause mortality observed [28]. Based on our results, we are suggesting the hypothesis that initiation of a DPP-4 inhibitor early in the treatment continuum for patients with T2DM may reduce the risk of CV complications in the long term. This hypothesis may be partly addressed by the ongoing VERIFY trial, a randomized 5-year study evaluating the durability of glycemic control with an early combination of vildagliptin and metformin, as in this trial MACEs are being adjudicated [29].
Our results are also of clinical importance as it has been shown that diabetes imposes a higher number of years of life lost because of CV disease in patients aged 40 and 50 years [12]: therefore, an early correction of risk factors as we observed in this study may contribute significantly to prevent MACE in younger population. Additionally, the findings from the present study support the comprehensive approach of CV risk factor reduction that not only focuses on glucose control but also on blood pressure and lipid management, in line with the recommendations of the T2DM guidelines [30].
Some limitations of this post hoc, hypothesis-generating exploratory analysis have been discussed earlier. Additional limitations are the average exposure to vildagliptin, which was approximately 52 weeks, so the results do not inform us of CV benefit with long-term vildagliptin therapy. The studies included in the pooled analysis were not powered to detect changes in cardio-metabolic parameters other than glycemia; nevertheless, the large pools of patients lend validity to the analysis. Although the analysis was adjusted for the use of CV medications at baseline for assessing changes in BP and lipids, the possible masking of the effect in those aged ≥ 65 years due to high usage of medications for prevention of secondary CV events or management of established complications cannot be ruled out.
Based on the findings from the current exploratory analysis, it can be hypothesized that in patients aged < 65 years with T2DM along with a low prevalence of CV disease and risk factors, treatment intensification with a DPP-4 inhibitor, vildagliptin, early in the treatment continuum improves key CV risk factors, which may lead to a reduction in the risk of major adverse CV events.
Acknowledgements
Sponsorship and article processing charges for this study were funded by Novartis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published. The authors acknowledge Ishita Guha Thakurta and Amit Garg of Novartis Healthcare Private Ltd., India, for medical writing support.
Disclosures
Marc Evans received financial support for consultancy from Novartis, Merck Sharp & Dohme Corp. and Novo Nordisk and has served on the speaker’s bureau for Novartis, Lilly, Boehringer lngelheim, Merck Sharp & Dohme Corp., Novo Nordisk, Janssen and Takeda. Plamen Kozlovski is an employee of and owns stocks in Novartis. Päivi M. Paldánius is an employee of and own stocks in Novartis. James E. Foley is an employee of and owns stocks in Novartis. Carmen Serban is an employee of and owns stocks in Novartis. Vaishali Bhosekar is an employee of Novartis. Angelo Avogaro is a member of the advisory panel in Merck Sharp & Dohme Corp., AstraZeneca, Boehringer lngelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, Takeda, Amgen Inc., Mylan, Bruno Farmaceutici and Novartis; is a board member of Merck Sharp & Dohme Corp., AstraZeneca, Novartis, Boehringer lngelheim, Sanofi, Takeda and Amgen Inc.; has provided consultancy for Merck Sharp & Dohme Corp., AstraZeneca, Novartis, Boehringer lngelheim, Sanofi, Novo Nordisk and Takeda; has received research support from AstraZeneca and Boehringer lngelheim; has served on the speaker’s bureau for Merck Sharp & Dohme Corp., AstraZeneca, Novartis, Boehringer lngelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, Takeda, Mylan and Bruno Farmaceutici.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. All study protocols were approved by an independent ethics committee or institutional review board. A written informed consent was collected from all participants to participate in the respective trials included in this pooled analysis.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Content
To view enhanced content for this article go to http://www.medengine.com/Redeem/8BCCF06053FC1668.
References
- 1.The Emerging Risk Factors Collaboration. Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Emerging Risk Factors Collaboration. Di Angelantonio ED, Gao P, et al. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311:1225–1233. doi: 10.1001/jama.2014.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccara F, Cohen A. Interplay of diabetes and coronary heart disease on cardiovascular mortality. Heart. 2004;90:1371–1373. doi: 10.1136/hrt.2004.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White WB, Kupfer S, Zannad F, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39:1267–1273. doi: 10.2337/dc16-0303. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes (2008). www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed 18 Apr 2016.
- 6.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Eng J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 7.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Eng J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer A, Dejager S, Foley JE, et al. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485–494. doi: 10.1111/j.1463-1326.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 10.McInnes G, Evans M, Del Prato S, et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17,000 patients. Diabetes Obes Metab. 2015;17:1085–1092. doi: 10.1111/dom.12548. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services (2014). https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 25 May 2017.
- 12.The Emerging Risk Factors Collaboration. Seshasai SR, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strain WD, Paldánius PM. Dipeptidyl peptidase-4 inhibitor development and post-authorisation programme for vildagliptin—clinical evidence for optimised management of chronic diseases beyond type 2 diabetes. Eur Endocrinol. 2017;13:62–67. doi: 10.17925/EE.2017.13.02.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pscherer S, Kostev K, Rockel T, Dworak M. HbA1c reduction in type 2 diabetes patients in clinical practice: comparison between vildagliptin and other DPP-4 inhibitors. Perfusion. 2011;24:206–211. [Google Scholar]
- 15.Evans M, Schweizer A, Foley JE. Blood pressure and fasting lipid changes after 24 weeks’ treatment with vildagliptin: a pooled analysis in > 2000 previously drug-naive patients with type 2 diabetes mellitus. Vasc Health Risk Manag. 2016;12:1–4. doi: 10.2147/VHRM.S112148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aryangat AV, Gerich JE. Type 2 diabetes: postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manag. 2010;6:145–155. doi: 10.2147/vhrm.s8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokhale M, Buse JB, Jonsson M, et al. No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase 4 inhibitors versus therapeutic alternatives. 2017. Diabetes Obes Metab. 2017;19:970–978. doi: 10.1111/dom.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 20.Gæde P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 21.Bain S, Druyts E, Balijepalli C, et al. Cardiovascular events and all-cause mortality associated with sulphonylureas compared with other antihyperglycaemic drugs: a Bayesian meta-analysis of survival data. Diabetes Obes Metab. 2017;19:329–335. doi: 10.1111/dom.12821. [DOI] [PubMed] [Google Scholar]
- 22.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®) Diab Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaccaro O, Masulli M, Bonora E, et al. Addition of either pioglitazone or a sulfonylurea in type 2 diabetic patients inadequately controlled with metformin alone: impact on cardiovascular events. A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:997–1006. doi: 10.1016/j.numecd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Eng J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 25.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Eng J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 28.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 29.Del Prato S, Foley JE, Kothny W, et al. Study to determine the durability of glycaemic control with early treatment with a vildagliptin-metformin combination regimen vs. standard-of-care metformin monotherapy—the VERIFY trial: a randomized double-blind trial. Diabet Med. 2014;31:1178–1184. doi: 10.1111/dme.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.