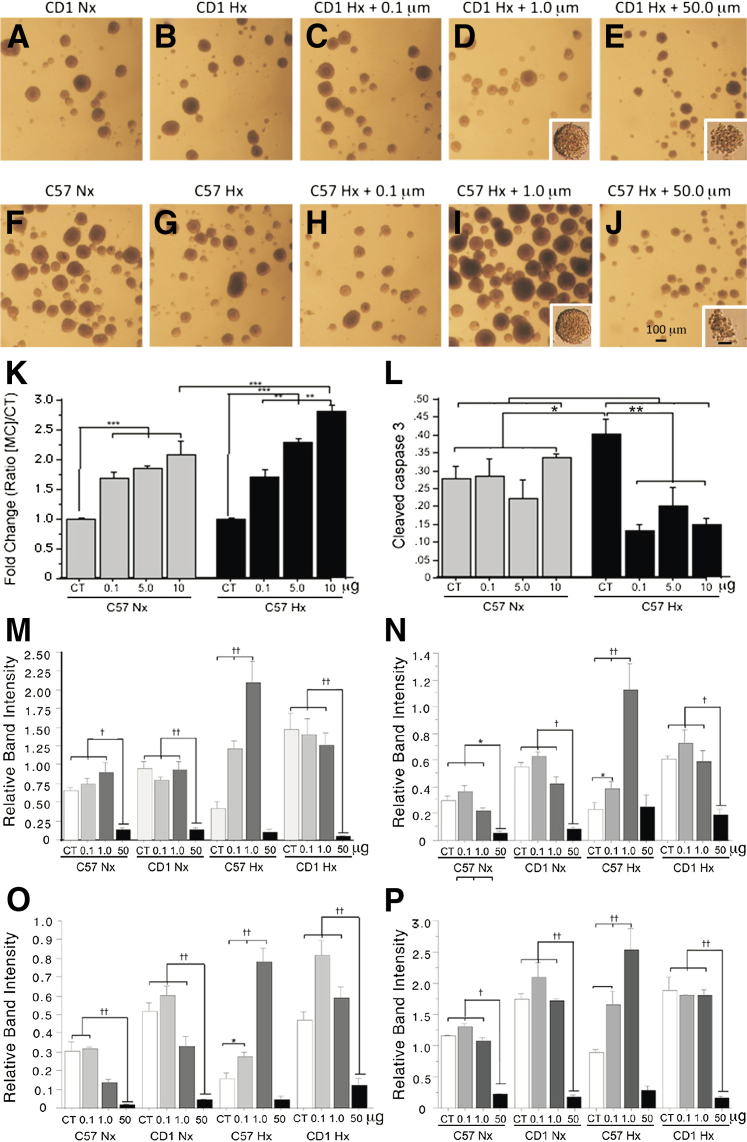
Figure 1.
Minocycline treatment elicits proliferation and reduces caspase 3 activation of reduced oxygen-cultured secondary neurosphere neural stem cell (NSC) cultures and increases in Sox10, HIF-1α, PCNA, and cleaved notch1 expression in reduced oxygen-cultured (HX) C57BL/6 NSC compared to normoxic (NX) cultures in a dose-specific fashion. Representative low-power micrographs illustrate the lack of responsiveness of CD1 secondary neurosphere cultures to reduced oxygen (10%) (A–D); whereas treatment with minocycline shows the increased number of neurospheres noted in hypoxic neurospheres cultures of C57BL/6 after treatment of 0.1 and 1.0 μmol/L minocycline (F–I). E and J and insets illustrate the loss of cohesiveness and crenated morphology of CD1 and C57BL/6 secondary neurospheres and cells after treatment with 50 μmol/L minocycline. Quantitation of neurospheres numbers (K) and cleaved caspase 3 (L) in cultures of C57BL/6 secondary neurospheres cultured under normoxic (20% O2) and reduced oxygen (10% O2) conditions, treated with 0.1, 5.0, and 10.0 μmol/L minocycline. Quantitation of Western blots of normoxic (20% O2) and reduced oxygen (10% O2) cultured C57BL/6 and CD1 NSC illustrating the differential induction of Sox10 (M), HIF-1α (N), cleaved notch1 (O), and PCNA (P) in the absence and presence of 0.1, 1.0, and 50 μmol/L minocycline. Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005; †P < 0.01, ††P < 0.001. CT, control added; HIF, hypoxia-inducible factor; MC, minocycline; NSC, neural stem cell; PCNA, proliferating cell nuclear antigen.