Abstract
Purpose:
The diagnosis of cancer and its treatment can make patients psychologically distressed. The purpose of this study is to evaluate the level of psychological distress and social functioning in cancer patients and to assess the association of these parameters with the quality of life (QOL) experienced by the patient.
Patients and Methods:
All cancer patients attending palliative care clinic who can understand and speak English or Tamil language were taken into the study. An interview technique with a questionnaire is used for data collection after informed consent. The questionnaire consisted of four sections, namely, demographic variables, general health questionnaire, WHO QOL-BREF, and SCARF social functioning index. All questionnaires were translated into the Tamil Language and were evaluated by the experts for content validity.
Results:
The median scores obtained are psychological distress = 44 (11–98), WHO QOL = 64 (36–117), and social function = 51 (29–79). Out of 251 patients, 30% had severe psychological distress, 25.6% had poor QOL, and 23.2% were with severely affected social function. Skilled laborers had better scores compared to unskilled laborers (P < 0.05). Family size (<2 children) had a positive impact on the QOL (P = 0.008). Patients from urban locales had better social functioning than rural counterpart (P = 0.047), but no difference was observed in distress level or QOL. Increased growth hormone distress score of the patients had a negative impact on both QOL (r = −0.522) and social function (r = −0.244). QOL correlated positively with social function (r = +0.247).
Conclusion:
Psychosocial stress associated with cancer and its treatment can impact the QOL and social functioning of the patient and needs to be addressed along with the cancer-directed therapy. Decreasing the symptom burden and distress level by palliative care intervention might improve the QOL and social function.
Keywords: Cancer, psychological distress, palliative care, quality of life, social functioning level
INTRODUCTION
Cancer is a disease involving intensive treatment with different modalities such as surgery, radiation, and chemotherapy. This along with the disease burden may makes patients with cancer psychologically distressed. The psychological and social issues are often overlooked by the physicians while attending a patient with cancer. Several studies have shown the stress associated with cancer treatment might have a role in progression of cancer[1,2,3,4] and cancer-related death.[5,6,7,8] The prevalence of psychosocial distress among cancer patients is estimated to be around 30%.[9] Although the impact of stress-modifying interventions on the outcome of cancer is not established,[1] there is a need to address the psychosocial issues in cancer patients in addition to the multimodality cancer-directed therapy to attempt to improve their quality of life (QOL). The purpose of this study is to evaluate the level of psychological distress and social functioning in cancer patients and to assess the association of these parameters with the QOL experienced by the patient.
PATIENTS AND METHODS
The population intended were cancer patients attending the palliative care clinic in our tertiary care cancer center who can understand and speak English or Tamil language. The criteria for inclusion were histopathologically confirmed malignancy, age more than 20 years, and ability to communicate effectively with the investigator either in Tamil or English language. Critically ill patients, those with hearing and speech defects, and pediatric age group were excluded from the study. The purpose of the study was explained, and oral consent was taken. A total of 251 patients could complete the questionnaire and were included in the study. The assurance was given to the study participants that anonymity of each individual will be maintained. Interview techniques were used to collect the data. The questions were read out to the patient (in their language of preference, i.e., Tamil or English) and the responses were marked in the questionnaire. The questionnaire consisted of four sections, namely, demographic and background variables of the patients, general health questionnaire, WHO QOL abbreviated study questionnaire (WHO QOL-BREF), and Scarf social functioning index.
The questionnaire consisted of five sections
Section 1: It consisted of tool to collect demographic variables of patients with cancer which include age, education, religion, occupation, marital status, age at marriage, type of marriage, the number of children, domicile, and treatment details.
Section 2: It is the general health questionnaire-28[10] and was used to measure the psychological distress of the cancer patients. The instrument consists of 28 items with four-point scale ranging from "not at all (0)" to "much more than usual (3)."
Information on the experience of symptoms of psychological distress will be obtained. The number of items in each element in the scale is as follows:
Somatic symptoms (7 items) 1, 2, 3, 4, 5, 6, 7
Anxiety and insomnia (7 items) 8, 9, 16, 18, 19, 20, 23
Social dysfunction (7 items) 10, 11, 12, 13, 14, 15, 17
Severe depression (7 items) 21, 22, 24, 25, 26, 27, 28.
Section 3: It had WHO QOL abbreviated survey questionnaire WHO QOL-BREF (1996). It is a 26-item generic questionnaire with 5-point scale ranging from "very bad (1)" to "very good (5)". It provides a short form of QOL.
Section 4: SCARF Social functioning index[11] consisted of tool to assess the functioning level of patients with cancer receiving the treatment. It includes four subsections. They are self-concern, occupational role, role in the family, and other social roles. Each section has four subsections, except the section on "other social roles" which has five subsections.
Self-concern includes self-care, personal belongings, personal space, eating practices, and health care
The occupational role includes regularity in occupational functioning, quality of occupation, quality of performance, and occupational interests
Role in the family includes marital role, role as a child, role as a parent, and family relationships
Other social roles include relationship with family members not living in the same home, relationship with friends, relationship with neighbors, colleagues at the place of work, and social activity groups.
Scoring procedure
WHO quality of life-BREF
A 5-point scale ranging from very poor (1) to very good (5) was used, the maximum score was 130. The scores are ranked as follows:
Very poor 1–26
Poor 27–52
Neither poor nor good 53–78
Good 79–104
Very good 105–130.
General health questionnaire
The instrument consists of 28 items with 4-point scale ranging from not all (0) to much more than usual (3). The scores are ranked as follows:
No distress – a score of 0
Mild distress – a score from 1 to 28
Moderate distress – a score from 28 to 56
Severe distress – a score from 57 to 84.
SCARF social functioning index
The scoring is made by rating each subcomponent from 1 to 5 and the sum of all is measured as a composite score. The scores are ranked as follows:
Mildly affected social functioning >60
Moderately affected social functioning 30–60
Severe affected social functioning < 30.
The questionnaires are standardized tools with the scoring method. All questionnaires were translated into the Tamil Language which was evaluated by the experts for content validity.
Statistics
Descriptive statistics was used for analyzing the data. The data are expressed in terms of frequency, measures of central tendency using mean with standard deviation, or median with range depending on the distribution of data. Kruskal–Wallis and Mann–Whitney–Wilcoxon tests were used to check the association of demographic parameters with scores. Spearman's rank correlation coefficients were calculated to check the association between the scores used in the study. The significance level was set at P < 0.05. All tests were done using IBM SPSS statistical software version 19 (IBM, USA).
RESULTS
The descriptive data are presented in Table 1. The median age was 52 years. The patients were grouped according to age, and approximately patients of age <40 years were 19%, 40–55 years were 44%, and >55 years were 37%. About 53.8% were males, and 46.2% were females. About 62% of the patients were illiterate and 53% were unemployed. Nearly, 76% of the patients belonged to the rural community, and 67% had more than two children. The median scores obtained are psychological distress = 44 (11–98), WHO QOL = 64 (36–117), and social function = 51 (29–79). About 62.4% responded as having moderate distress and 30% had severe distress, 25.6% experienced poor QOL, and 23.2% had severely affected social functioning. The Frequency of scores among the patients is presented in Table 2 and illustrated in Diagram 1.
Table 1.
Scores observed among different patient subgroups
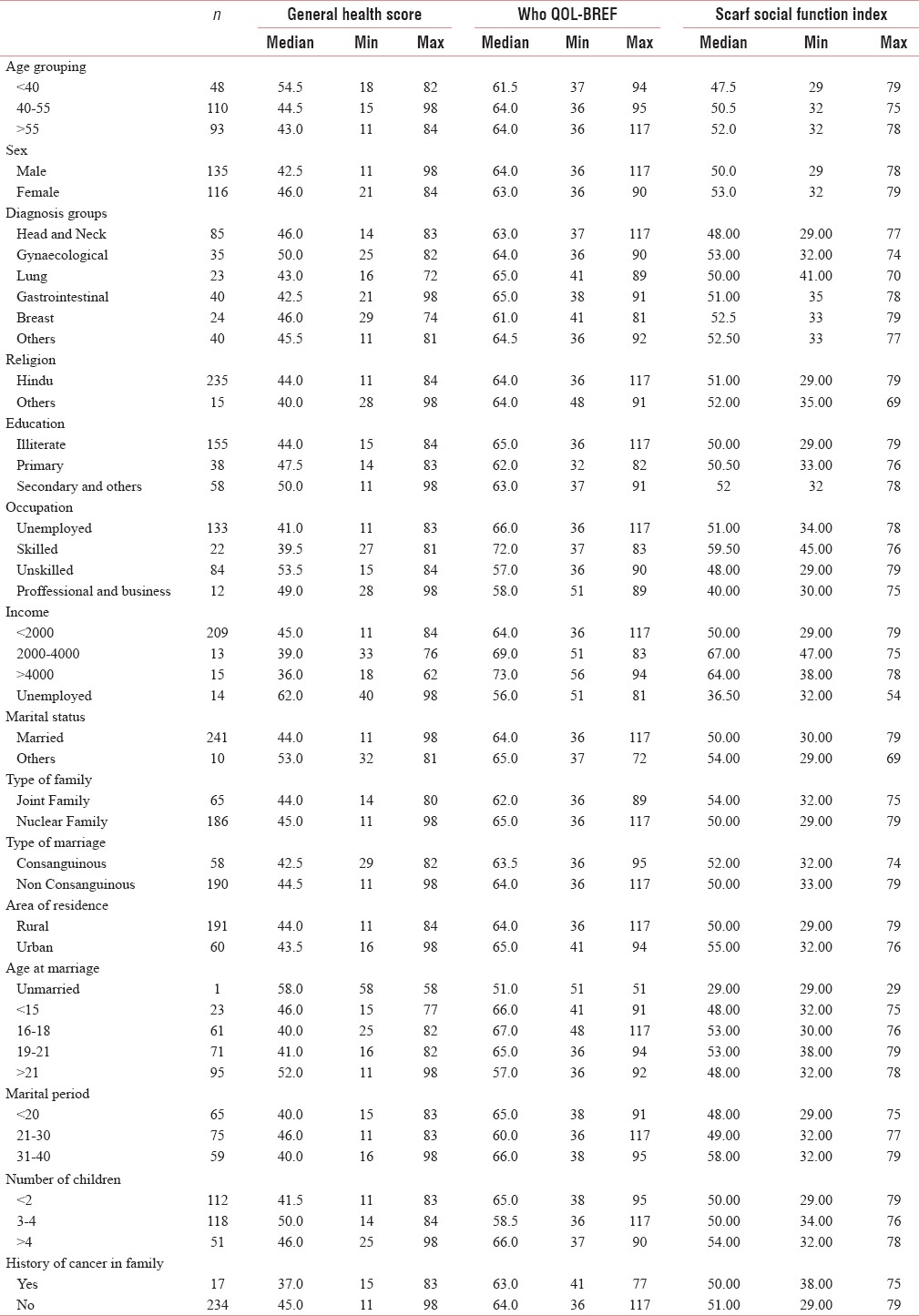
Table 2.
Frequency of scores among the patients
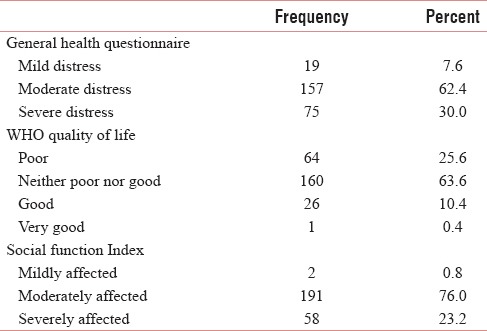
Diagram 1.
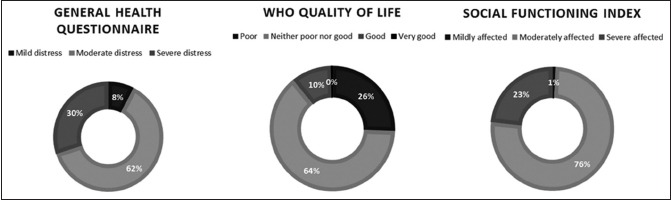
Diagram representing the distribution of scores among the patients
The relationship between patient characteristics and scores
General health questionnaire
Age has no influence on the distress level. There was no significant difference seen in distress experienced among different age groups (P = 0.108). Females tend to have more distress than males, but this difference has not reached statistical significance (46 vs. 42.5; P = 0.07). Unskilled laborers had more distress than skilled laborers (P = 0.007). Patients married for more than 40 years had significantly higher scores than the other groups (P = 0.001). There was no difference observed in distress levels among different sites of cancer, the number of children in the family.
WHO quality of life-BREF
There was a significant difference in the QOL with respect to occupation. Skilled laborers had the significantly better median quality of life scores than unskilled and unemployed (P < 0.001). Family size (<2 children) had a positive impact on the QOL (P = 0.008). There was no relation observed with age, site of cancer, or education.
SCARF social function index
Skilled workers had better social function index than unskilled and unemployed. Patients living in urban locales had better social function scores than rural counterparts. There was no difference in age groups or education.
Relationship between the scores
Increased growth hormone distress score of the patients had a negative impact on both QOL (r = −0.522) and social function (r = −0.244). QOL correlated positively with social function (r = +0.247).
DISCUSSION
In this study, we have evaluated the relationship between patient distress level, social function status, and QOL. At least a quarter of the patients responded that they experienced severe distress, have a poor QOL, and function poorly in the society. We have observed a correlation between these scores. The distress level experienced correlated negatively with social function status implying that the patients who experienced higher distress had a poor social function. Similarly, patients with higher distress levels had worse scores on WHO QOL questionnaire. The QOL correlated positively with social function indicating that patients with good QOL had better social function status. Unlike what was seen in a study by Mystakidou et al.,[12] our study did not show any relationship of age and education with distress or QOL. The diagnosis of cancer and the toxicity involved in its treatment may cause patients to experience psychological distress. We observed that these patients fail to fulfill their role as a member of family and society. We have seen that the unskilled laborers had worse scores in all three questionnaires. The disease might have prevented them to resume their work involving physical strength. Failure to provide to the family might have aggravated the distress and reduced the QOL. Hence, the improvement in QOL is not possible by cancer treatment alone but by alleviating the distress associated with the disease.
It has been in shown in studies that psychosocial stress has a role in cancer progression by its impact on mechanisms of immune regulation, angiogenesis, and invasion.[1] A study in ovarian cancer patients has shown that social support is related to lower levels of VEGF both in serum and tumor tissue.[13] In another study, ovarian cancer patients with poor social support expressed higher levels IL-6 which is an angiogenic factor produced by tumor cells that disrupt the equilibrium between pro- and anti-angiogenic factors.[14] There is an evidence of association between dysregulations in diurnal cortisol secretion and diminished QOL, greater functional disability, fatigue, and poorer outcomes in breast cancer patients.[15,16,17] Thus, the above-mentioned studies indicate that the stress experienced by the patients can affect the outcome of cancer. The role of psychosocial intervention in decreasing pain and anxiety,[18] improving QOL,[19,20,21] and ability to complete the therapy has been shown but whether it can affect cancer progression and survival has not been established.[19,20,21,22,23] Several reports have also shown the relationship between distress level and QOL[12,24] consistent with what we have observed in our study. Although the evidence is not convincing that any psychosocial intervention to improve the distress and QOL has any role in improving cancer outcomes, we believe that attempt should be made to reduce the distress and improve QOL by counselling and palliative care. The aim of cancer therapy should not only be prolonging life but also to maintain health as its holistic definition.
Limitations of this study include a population of a single institution with possible institutional bias, and the effect of the burden of disease on QOL and distress has not been evaluated. Although we attempted to compare the patient characteristics with the scores, conclusions cannot be drawn as it is an observational cohort study and has unequal distribution among patient subgroups. The strengths of the study are heterogeneous population with a variety of disease groups and excellent participation of the study participants in responding to the questionnaire.
CONCLUSION
Psychosocial stress associated with cancer and its treatment is often overlooked by the treating physicians. It can impact the QOL and social functioning of the patient and needs to be addressed along with the cancer-directed therapy. As there is a significant correlation between psychosocial distress and QOL experienced, interventions such as holistic palliative care and social support aiming to reduce the patients’ distress could result in improved QOL and better social functioning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the nursing staff and medico-social workers of Palliative Care Unit, JIPMER, for their help in conducting this project.
REFERENCES
- 1.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094–9. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–13. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 6.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol. 2007;25:2397–405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 7.Everson SA, Goldberg DE, Kaplan GA, Cohen RD, Pukkala E, Tuomilehto J, et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med. 1996;58:113–21. doi: 10.1097/00006842-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94:2719–27. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 9.Herschbach P, Book K, Brandl T, Keller M, Lindena G, Neuwöhner K, et al. Psychological distress in cancer patients assessed with an expert rating scale. Br J Cancer. 2008;99:37–43. doi: 10.1038/sj.bjc.6604420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg DP, Hillier VF. A scaled version of the general health questionnaire. Psychol Med. 1979;9:139–45. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 11.Padmavathi R, Thara R, Srinivasan L, Kumar S. Scarf social functioning index. Indian J Psychiatry. 1995;37:161–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Mystakidou K, Tsilika E, Parpa E, Pathiaki M, Galanos A, Vlahos L, et al. The relationship between quality of life and levels of hopelessness and depression in palliative care. Depress Anxiety. 2008;25:730–6. doi: 10.1002/da.20319. [DOI] [PubMed] [Google Scholar]
- 13.Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–46. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–13. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 17.Touitou Y, Lévi F, Bogdan A, Benavides M, Bailleul F, Misset JL, et al. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin Oncol. 1995;121:181–8. doi: 10.1007/BF01198101. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–26. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 19.Coyne JC, Stefanek M, Palmer SC. Psychotherapy and survival in cancer: The conflict between hope and evidence. Psychol Bull. 2007;133:367–94. doi: 10.1037/0033-2909.133.3.367. [DOI] [PubMed] [Google Scholar]
- 20.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–84. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel D. Effects of psychotherapy on cancer survival. Nat Rev Cancer. 2002;2:383–9. doi: 10.1038/nrc800. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann PG. Psychosocial interventions in breast cancer: To light a candle. Cancer. 2009;115:5617–9. doi: 10.1002/cncr.24659. [DOI] [PubMed] [Google Scholar]
- 23.Stefanek ME, Palmer SC, Thombs BD, Coyne JC. Finding what is not there: Unwarranted claims of an effect of psychosocial intervention on recurrence and survival. Cancer. 2009;115:5612–6. doi: 10.1002/cncr.24671. [DOI] [PubMed] [Google Scholar]
- 24.Götze H, Brähler E, Gansera L, Polze N, Köhler N. Psychological distress and quality of life of palliative cancer patients and their caring relatives during home care. Support Care Cancer. 2014;22:2775–82. doi: 10.1007/s00520-014-2257-5. [DOI] [PubMed] [Google Scholar]


