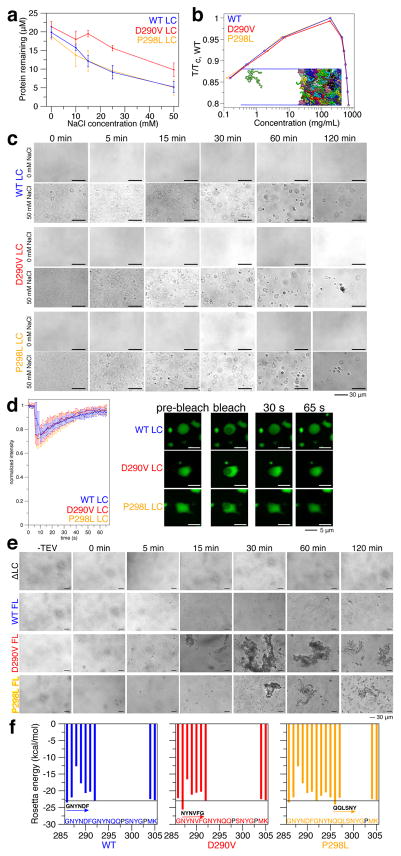
Figure 3. hnRNPA2 undergoes LLPS and mutations induce aggregation.
a) With increasing salt, hnRNPA2 LC WT, D290V, and P298L show decreasing protein remaining in the supernatant after sedimentation of droplets. WT and P298L are similar, while D290V has comparably more protein remaining, indicating decreased propensity for phase separation. Data represent mean ± SD.
b) Coexistence curve from coarse-grained slab simulations of hnRNPA2 LC with temperature normalized by the critical temperature of WT. Insert shows snapshot of a region of the simulation box containing both low and high protein density phases.
c) DIC micrographs show hnRNPA2 LC WT, D290V, and P298L form liquid droplets. Over time, D290V droplets seed persistent aggregates with fibrous structure. P298L forms non-spherical clusters consistent with aggregation. In the absence of salt (NMR conditions), no micron-sized species are observed. Scale bar represents 30 μm.
d) Fluorescence recovery after photobleaching experiments show that freshly made WT, D290V, and P298L hnRNPA2 LC droplets recover similarly from partial droplet bleach, consistent with liquid behavior. Scale bar represents 5 μm.
e) Full-length WT hnRNPA2 undergoes LLPS after cleavage of a solubility tag. At the same conditions, D290V quickly forms aggregates. Over time, WT droplets fuse and grow while D290V aggregates grow larger. P298L forms droplets initially but aggregates over time. Deletion of LC domain (residues 1–189, ΔLC) prevents both LLPS and aggregation. Scale bar, 30 μm.
f) D290V has amyloid zipper propensity at the NYNVFG peptide exceeding an estimated Rosetta energy threshold for amyloid formation (line at −23 kcal/mol), while P298L shows both increased zipper propensity at the QQLSNY peptide and extended zipper-favoring region (peptides starting at residues 293 to 297).
See also Figure S4.