Abstract
Background/Objective
We investigated the effect of plantar cutaneous inputs on the postural sway during quiet standing in older adults.
Methods
Eight healthy elderly individuals (age 72.3 ± 4.4 years) stood on a force platform for 30 seconds without and with mechanical facilitation of sensation from the forefoot (a small coin-shaped object under the sole), and their eyes closed. Ellipse area and mean velocity of center of pressure, rambling and trembling trajectories in the anterior–posterior (AP) and medial–lateral directions were analyzed.
Results
The ellipse area in the stimulation condition was significantly reduced as compared to the control condition. Significant decreases were also observed in the stimulation condition for the velocity of the center of pressure in both AP and medial–lateral directions and for velocity of the trembling trajectory in the AP direction.
Conclusion
The findings indicate that mechanical facilitation of sensation on the plantar soles enhanced postural stability in older adults. The results show that plantar cutaneous inputs provide information that leads to reduced postural sway in healthy older adults. This could have implications in clinical and rehabilitative areas.
Keywords: Age, Balance, Postural control, Sole stimulation
Introduction
Somatosensory information is important to the control of the vertical posture. A reduced ability to detect or attend to the sensory information impairs the ability to detect changes in upright standing and increases the incidence of falls. During healthy aging, the deterioration in balance performance boosts the potential for fall-related injury and reduced function.1
Plantar cutaneous mechanoreceptors represent the direct interface between the body and the ground. The fast and slow adapting cutaneous mechanoreceptors are highly sensitive to the forces applied to the sole of the foot and they provide reliable information about the direction and amplitude of the center of pressure (COP).2, 3 Studies have found changes in receptor morphology with age, such as reduction in receptor density and elasticity, and a slower nerve conduction.4 This decrease of plantar cutaneous sensitivity can lead to a degradation of postural control and contribute to the increased incidence of falls and injuries in older adults.5
Plantar inputs, however, enhance the detection and transmission of weakened cutaneous signals. For instance, standing on spike insoles,6 pins,7 textured surfaces,8, 9 or on a tubing located on a plantar-surface boundaries10 were found to facilitate the sensation and to markedly reduce the postural sway. Therefore, the cutaneous inputs appear to be a good candidate for improving the postural stability in older adults.
Surprisingly, the use of a single and small object generating permanent stimulus to the sole of the foot was not studied, and the importance of this type of stimulation remains very much unexplored. Moreover, none of the available publications have used rambling–trembling analysis to study the effect of cutaneous inputs on postural stability. Thus, the present study was designed to test this possibility specifically. Our analysis decomposed the commonly reported COP to reflect its association with supraspinal (rambling component) and peripheral (mechanical and reflex, trembling component) mechanisms.11, 12 More specifically, in order to explore the influence of the cutaneous inputs alone, we asked participants to stand with their eyes closed to suppress the vision information. We hypothesized that postural stability would increase in older adults with mechanical facilitation of sensation on the plantar soles.
Methods
Participants
Eight older individuals (4 men and 4 women; mean ± standard deviation age = 72.3 ± 4.4 years, weight = 59.1 ± 9.2 kg, and height = 160 ± 5.2 cm) volunteered to participate in the study. All were healthy and none had a history of central or peripheral neurological disorders or problems related to movements of the spinal column (e.g., significant arthritis or musculoskeletal abnormalities). The participants gave informed consent to take part in the study, which conforms to the standards set in the Declaration of Helsinki.
Apparatus
We used a force platform (Model BP400600-2000; AMTI, Watertown, MA, USA) to record the three orthogonal components of the force (along the direction of gravity FZ, parallel to the ground in the sagittal plane FX, and parallel to the ground in the frontal plane FY) and three components of the moment (the moment about the sagittal–horizontal axis MX, the moment about the frontal–horizontal axis MY, and the moment about the vertical axis of the body MZ). The signals from the force platform were digitized at the sampling frequency of 1000 Hz with a 16-bit resolution. A Dell 3.3 GHz computer (Dell Inc., Roundrock, TX, USA) was used to control the experiment and collect the data using Chart version 5.5.6 (AD Instruments, Milford, MA, USA).
Stimulus
Mechanical facilitation of sensation was a coin-shaped aluminum alloy (diameter 26.5 mm). It was placed on the force plate while participants stood barefoot approximately at the junction of the anterior third and posterior two thirds of the participant's sole. The height of the stimulation was set individually according to each participant's sole detection threshold. This threshold was found prior to the experimental session. Setting the threshold involved raising the stimulation height first and then decreasing it until the participant could only feel a tactile superficial sensation. This sole detection threshold was verified by repeating this procedure twice while the participant stood in an upright position.
Procedure
Participants were asked to stand barefoot on the force platform during 30 seconds without and with stimuli of both soles, and their eyes closed. They stood with their feet positioned side-by-side at a comfortable width (shoulder width), and arms placed in a relaxed position at the body's sides. Three trials were performed in each condition with 1-minute breaks between trials. The order in which each condition was presented in each trial was randomized across participants. Foot position was marked on the platform and repeated across trials.
Data processing
We processed all signals offline by using MATLAB version 4.16 (R2011b; The MathWorks, Natick, MA, USA) software packages. Signals from the force plate were filtered with a 20-Hz low-pass, second-order, zero-lag Butterworth filter. Coordinates of the COP in the anterior–posterior (AP) and medial–lateral (ML) directions (COPAP and COPML, respectively) were calculated using the following approximations:
| COPAP = (−My − Fx × d)/Fz | (1) |
| COPML = (Mx − Fy × d)/Fz | (2) |
where coefficient d is the distance from the origin of the platform to its surface (45 mm in our force platform).
Once we calculated COPAP and COPML, we band-pass filtered (0.04–10.00 Hz) the COP data using a second-order, zero-lag Butterworth filter. To assess the dispersion of the COP, ellipses were fit to the data and area of the ellipse enclosing 95% of COP movement was calculated. The two main axes of the ellipse were found by calculating the eigenvalues of the covariance matrix between the AP and ML data.13 The first eigenvector of the covariance matrix was the direction of the major axis and the corresponding largest eigenvalue was the variance along this axis. The second eigenvector, which was orthogonal to the first eigenvector, defined the direction of the minor axis and the corresponding eigenvalue was the variance along this axis.
Decomposition of each COP time series into the rambling (the motion of an instant equilibrium point about which the body's equilibrium is maintained, RM) and trembling (the oscillation of COP around the reference point, TR) components was done for the AP and ML directions separately as described by Zatsiorsky and Duarte.11, 12 Instantaneous equilibrium points were identified as COP coordinates when the horizontal force was zero. The RM trajectory was established as the interpolation of those points using a cubic spline function. The TR trajectory was determined as the difference between COP and RM trajectories.
Statistical analysis
Mean values of the main outcome variables calculated from the three 30-second trials at each of the conditions were included for further statistical analysis. For statistical analyses, the SPSS version 15.0J for Windows (SPSS Inc., Chicago, IL, USA) was used. The Shapiro–Wilk test was carried out to assess the normality of the data. A paired t test was used to determine the significant differences between conditions. Level of significance was set at α = 0.05.
Results
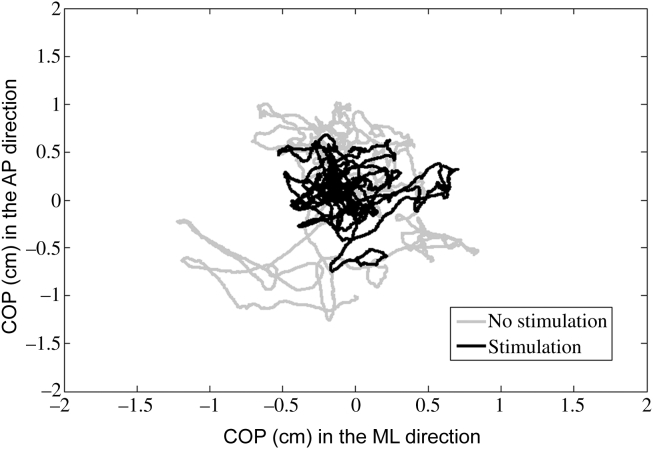
The Shapiro–Wilk test showed that all the data produced were normally distributed (Table 1). The COP trajectories during each condition for a representative participant are illustrated in Figure 1. Postural sway displacement in the sole stimulation condition was smaller than in the no stimulation condition in range. Detailed statistical analyses of each variable of 95% confidence ellipse are separately presented below for each condition. Table 2 shows the means and standard errors of each variable. It can be seen that ellipse area in the stimulation condition was significantly reduced (p = 0.025) as compared to the control condition and the major axis reduction approached significance (p = 0.064).
Table 1.
Normality test for center of pressure confidence ellipse area and trajectories in each condition.
| Shapiro–Wilk test (p) |
||
|---|---|---|
| No stimulation | Stimulation | |
| Area | 0.114 | 0.072 |
| Major axis | 0.644 | 0.565 |
| Minor axis | 0.088 | 0.152 |
| COP_AP | 0.426 | 0.858 |
| COP_ML | 0.080 | 0.057 |
| RM_AP | 0.669 | 0.257 |
| RM_ML | 0.321 | 0.052 |
| TR_AP | 0.664 | 0.561 |
| TR_ML | 0.060 | 0.585 |
Figure 1.
Anterior–posterior (AP) center of pressure (COP) plotted versus medial–lateral (ML) COP for each time series for a representative participant when standing quietly with eyes closed in the stimulation condition (gray line) and in the control condition (black line). A 95% confidence ellipse was fit to these traces and area was calculated. Ellipses have been omitted for clarity.
Table 2.
Means and standard errors (SE) of center of pressure confidence ellipse area for the different conditions.a
| No stimulation |
Stimulation |
p | |||
|---|---|---|---|---|---|
| Mean ± SE | 95% CI | Mean ± SE | 95% CI | ||
| Area (cm2) | 4.61 ± 1.03 | 2.33–6.89 | 3.50 ± 0.92 | 1.64–5.35 | 0.025 |
| Major axis (cm) | 1.63 ± 0.20 | 1.20–2.07 | 1.38 ± 0.20 | 0.94–1.82 | 0.064 |
| Minor axis (cm) | 0.85 ± 0.12 | 0.59–1.12 | 0.75 ± 0.09 | 0.54–0.96 | 0.100 |
CI = confidence interval.
Significant differences between conditions are in bold.
Mean velocity of the COP component was calculated as total distance divided by the duration of the measurement. A summary of the results across the conditions for COP, RM, and TR is presented in Table 3. During the sole stimulation condition, participants showed a general tendency to move the COP at a lower velocity over a smaller amplitude. This general trend was more pronounced in the COP trajectories as compared to the RM and TR components. Significant differences between the stimulation and control conditions were observed for the velocity of the COP in both AP (p = 0.036) and ML (p = 0.012) directions and for velocity of the TR in the AP direction (p = 0.017). All mentioned sway parameters showed a significant decrease during the stimulation stance as compared to the control stance. The largest mean velocity occurred in the TR in the AP direction. This large mean velocity reflects the long sway path travelled over 30 seconds by participants in the control condition. In both conditions, mean velocity was decreased compared with the control condition, even if statistical significance was not observed.
Table 3.
Means and standard errors (SE) of the velocities of the center of pressure component between conditions.a
| No stimulation |
Stimulation |
p | |||
|---|---|---|---|---|---|
| Mean ± SE | 95% CI | Mean ± SE | 95% CI | ||
| COP_AP | 1.34 ± 0.18 | 0.94–1.73 | 1.24 ± 0.14 | 0.92–1.55 | 0.036 |
| COP_ML | 0.94 ± 0.18 | 0.68–1.27 | 0.85 ± 0.13 | 0.57–1.13 | 0.012 |
| RM_AP | 0.43 ± 0.06 | 0.30–0.56 | 0.42 ± 0.05 | 0.30 –0.54 | 0.779 |
| RM_ML | 0.33 ± 0.04 | 0.24–0.42 | 0.41 ± 0.07 | 0.24–0.57 | 0.674 |
| TR_AP | 1.53 ± 0.18 | 1.13–1.93 | 1.42 ± 0.15 | 1.09–1.75 | 0.017 |
| TR_ML | 1.16 ± 0.14 | 0.75–1.57 | 1.12 ± 0.14 | 0.82–1.42 | 0.779 |
CI = confidence interval.
Significant differences between conditions are in bold.
Discussion
The purpose of the present study was to explore the effect of a single object generating permanent plantar cutaneous inputs on the postural stability in older adults. It was inferred from quantitative COP measures in two conditions. In support of our study hypothesis, the COP findings indicated that plantar cutaneous inputs led to an increased capacity to maintain postural stability during quiet stance with eyes closed. Our results suggest that the use of a single and small object generating permanent stimulus to the sole of the forefoot could be a potential aspect to be taken into account and controlled when considering postural balance in older adults. We next interpret the significance of our findings for COP measures in relation to their modifiability based on plantar cutaneous inputs.
Upright human posture is considered to be inherently unstable due to the difficulty in maintaining the high center of gravity on the relatively small base of support provided by the feet.14 The COP measurements are commonly used output measures of postural sway, as they reflect the sway of the body and forces used to maintain the center of gravity within the support base.15, 16 Previous studies have found that somatosensory information from the foot soles is the critical sensory information for control of posture during standing.6, 7 Palluel et al17 found that spike insoles can decrease COP area of postural sway in older people. Our results showed that COP ellipse area in the stimulation condition was significantly reduced as compared to the control condition. We infer that the large area of postural sway in the control condition reflects the ineffectiveness of postural control and deficits in fine tuning movements that may be related to poor use of somatosensory information associated with aging. This speculation is consistent with previous findings that demonstrate COP shifts larger than the normal ellipse area when somatosensory feedback is delayed peripherally.18 By contrast, one could assume that the reduced postural sway area in the stimulation condition may reflect increased somatosensory feedback in the postural control loop and accurate detection of the spatial representation of body posture in this condition.
In terms of the mean velocity of COP, significant differences between the stimulation and control conditions were observed in both AP and ML directions. The velocities were significantly decreased in the stimulation condition. These results are consistent with previous studies.7, 18 The finding that TR component in the AP direction was influenced by sole stimulation is based on the equilibrium-point hypothesis of motor control.19 RM and TR are considered to reflect the association with supraspinal (RM) and peripheral (mechanical and reflex, TR) mechanisms. That is, RM is related to supraspinal processes that are involved in the control of the migration of the resting position of the COP, while TR is a result of the action of spinal reflexes and changes in the intrinsic mechanical properties of the muscles and joints.11, 12 In comparison with the control condition, TR decreased when the sole stimulation was presented. The drop in TR may be interpreted as the tactile information from the soles contributing to body posture awareness and spatial representation of the pressure distribution under feet soles. Zatsiosky and Duarte12 found that TR highly (and negatively) correlates with the horizontal ground reaction force. When the COP deviates from the instant equilibrium position, a horizontal force is triggered to correct the deviation. In our study, TR decrease suggests intrinsic stiffness of the lower extremity muscles generate the corrective force. In the previous study, increased agonist–antagonist co-contraction patterns in older adults have been shown to contribute to the challenges of maintaining vertical posture.20 Increased co-contraction may affect both peripheral mechanical properties of the joints as well as reflex effects. The drop in TR leads us to believe that improved body stability is achieved by decreasing the level of activation of muscle groups located around the ankle and hip joints, which, consequently, could decrease the oscillations about the joints.
Our results are important for understanding how older adults may benefit from a sole stimulation for postural stability in stance. In this study, the use of a single and small object generating permanent stimulus to the sole of the foot could be argued as novelty. This mechanical facilitation of sensation is not heavy and can be located on the sole. Our results suggest that a single sole stimulation could effectively modify standing balance in older adults.
We would like to mention some limitations that might have influenced the outcomes of the current study. First, a relatively small sample size limited statistical power of the study. Second, the choice of older participants means that the results may not be generalized to young individuals. It is possible that the type of stimulation could also be important for giving valuable information to enhance postural stability in young individuals. We will try to overcome these in our future studies.
Taken together, the findings of this study indicate that mechanical facilitation of sensation on the plantar soles enhanced postural stability in older adults. The results show that plantar cutaneous inputs provide information that leads to reduced postural sway in healthy older adults. This could have implications in clinical and rehabilitative areas.
Conflicts of interest
No conflicts of interest are declared.
Funding/support
This work was supported by the National Natural Science Foundation of China (Grant No. 31371207), the Natural Science Foundation of Tianjin (Grant No. 14JCYBJC43300), and the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research (C) No. 23500681).
References
- 1.Shumway-Cook A., Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 2.Fiolkowski P., Brunt D., Bishop M. Does postural instability affect the initiation of human gait? Neurosci Lett. 2002;323:167–170. doi: 10.1016/s0304-3940(02)00158-1. [DOI] [PubMed] [Google Scholar]
- 3.Nurse M.A., Nigg B.M. Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech (Bristol, Avon) 1999;14:667–672. doi: 10.1016/s0268-0033(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 4.Kenshalo D.R., Sr. Somesthetic sensitivity in young and elderly humans. J Gerontol. 1986;41:732–742. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- 5.Menz H.B., Morris M.E., Lord S.R. Foot and ankle risk factors for falls in older people: a prospective study. J Gerontol A Biol Sci Med Sci. 2006;61:866–870. doi: 10.1093/gerona/61.8.866. [DOI] [PubMed] [Google Scholar]
- 6.Palluel E., Olivier I., Nougier V. The lasting effects of spike insoles on postural control in the elderly. Behav Neurosci. 2009;123:1141–1147. doi: 10.1037/a0017115. [DOI] [PubMed] [Google Scholar]
- 7.Maurer C., Mergner T., Bolha B. Human balance control during cutaneous stimulation of the plantar soles. Neurosci Lett. 2001;302:45–48. doi: 10.1016/s0304-3940(01)01655-x. [DOI] [PubMed] [Google Scholar]
- 8.Hatton A.L., Dixon J., Rome K. Standing on textured surfaces: effects on standing balance in healthy older adults. Age Aging. 2011;40:363–368. doi: 10.1093/ageing/afr026. [DOI] [PubMed] [Google Scholar]
- 9.Qiu F., Cole M.H., Davids K.W. Enhanced somatosensory information decreases postural sway in older people. Gait Posture. 2012;35:630–635. doi: 10.1016/j.gaitpost.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Maki B.E., Perry S.D., Norrie R.G. Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M281–M287. doi: 10.1093/gerona/54.6.m281. [DOI] [PubMed] [Google Scholar]
- 11.Zatsiosky V.M., Duarte M. Instant equilibrium point and its migration in standing tasks: rambling and trembling components of the stabilogram. Motor Control. 1999;3:28–38. doi: 10.1123/mcj.3.1.28. [DOI] [PubMed] [Google Scholar]
- 12.Zatsiosky V.M., Duarte M. Rambling and trembling in quiet standing. Motor Control. 2000;4:185–200. doi: 10.1123/mcj.4.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Duarte M., Zatsiorsky V.M. Effects of body lean and visual information on the equilibrium maintenance during stance. Exp Brain Res. 2002;146:60–69. doi: 10.1007/s00221-002-1154-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Asaka T. Muscle synergies involved in shifts of the center of pressure while standing on a narrow support. Brain Res Bull. 2008;76:16–25. doi: 10.1016/j.brainresbull.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Chiari L., Bertani A., Cappello A. Classification of visual strategies in human postural control by stochastic parameters. Hum Mov Sci. 2000;19:817–842. [Google Scholar]
- 16.Wang Y., Watanabe K., Asaka T. Muscle synergies in preparation to a step made with obstacle in elderly individuals. J Neuroeng Rehabil. 2015;12:10. doi: 10.1186/s12984-015-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palluel E., Nougier V., Olivier I. Do spike insoles enhance postural stability and plantar-surface cutaneours sensitivity in the elderly? Age. 2008;301:53–61. doi: 10.1007/s11357-008-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horak F.B., Macpherson J.M. Postural orientation and equilibrium. In: Shepard J., Rowell L., editors. Handbook of Physiology. Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- 19.Feldman A.G., Levin M.F. Positional frames of reference in motor control: their origin and use. Behav Brain Sci. 1995;18:723–806. [Google Scholar]
- 20.Woollacott M.H., Shumway-Cook A. Changes in posture control across the life span—a systems approach. Phys Ther. 1990;70:799–807. doi: 10.1093/ptj/70.12.799. [DOI] [PubMed] [Google Scholar]