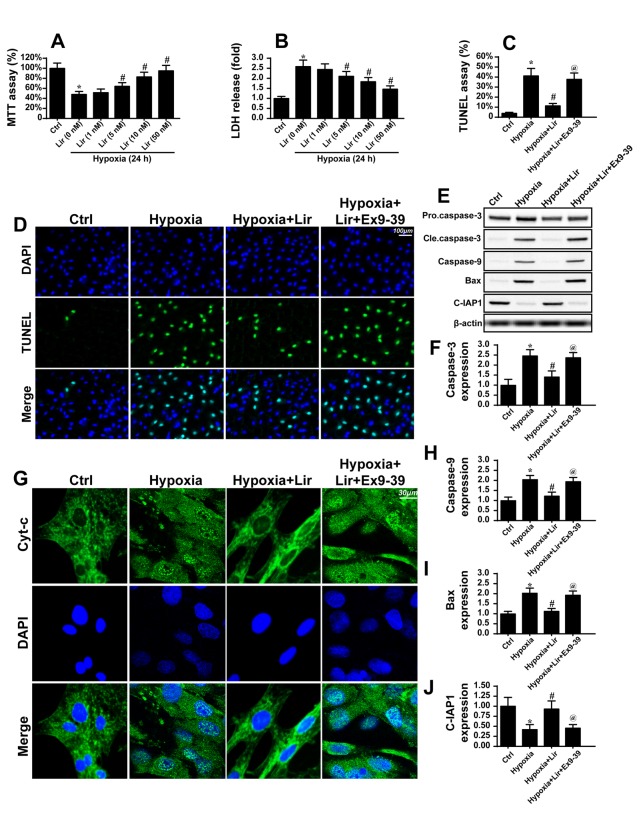
Figure 2.
Liraglutide reduces cardiomyocyte death via inhibition of mitochondrial apoptosis. The hypoxia model of H9C2 in vitro was used to mimic chronic cardiac injury. H9C2 cells were cultured under hypoxic condition for ~48 h. (A) MTT and (B) LDH assays were used to detect the cell viability. Liraglutide was able to sustain H9C2 viability in a dose-dependent manner under 48 h of hypoxia. *P<0.05 vs. Ctrl group; #P<0.05 vs. Lir (0 nM) group. Since the minimum concentration of liraglutide, which significantly promoted H9C2 viability under hypoxia was 5 nM, this concentration was used in the following experiments. (C) A TUNEL assay was used to observe the cellular apoptosis and (D) representative images are presented. Ex9-39, a water-soluble GLP-1 receptor antagonist was used to inhibit the action of liraglutide. *P<0.05 vs. Ctrl group; #P<0.05 vs. hypoxia group; @P<0.05 vs. hypoxia + Lir group. (E) Western blotting was used to detect alterations in the expression of proteins associated with mitochondrial apoptosis. (F) The protein expression of Caspase3 was quantified. (G) Immunofluorescence analysis of cyt-c localization. Chronic hypoxia induced the cyt-c leakage from mitochondria and trafficking into the nucleus. The protein expression levels of (H) caspase9, (I) Bax and (J) c-IAP1 were quantified. Liraglutide was able to reduce the expression of apoptotic proteins, and this effect was reversed by Ex9-39. *P<0.05 vs. Ctrl group; #P<0.05 vs. hypoxia group; @P<0.05 vs. hypoxia + Lir group. Lir, liraglutide; Ctrl, control; cle, cleaved; LDH, lactate dehydrogenase; TUNEL, terminal deoxynucleotidyl-transferas-mediated dUTP nick end labeling; Ex9-39, exendin 9–39; Bax, apoptosis regulator BAX; c-IAP1, baculoviral IAP repeat-containing protein 2; cyt-c, cytochrome c.