Abstract
4-Hydroxy-3-methoxybenzaldehyde (vanillin), contained in a number of species of plant, has been reported to display beneficial effects against brain injuries. In the present study, the impact of vanillin on scopolamine-induced alterations in cognition and the expression of DNA binding protein inhibitor ID-1 (ID1), one of the inhibitors of DNA binding/differentiation proteins that regulate gene transcription, in the mouse hippocampus. Mice were treated with 1 mg/kg scopolamine with or without 40 mg/kg vanillin once daily for 4 weeks. Scopolamine-induced cognitive impairment was observed from 1 week and was deemed to be severe 4 weeks following the administration of scopolamine. However, treatment with vanillin in scopolamine-treated mice markedly attenuated cognitive impairment 4 weeks following treatment with scopolamine. ID1-immunoreactive cells were revealed in the hippocampus of vehicle-treated mice, and were hardly detected 4 weeks following treatment with scopolamine. However, treatment with vanillin in scopolamine-treated mice markedly restored ID1-immunoreactive cells and expression 4 weeks subsequent to treatment. The results of the present study suggested that vanillin may be beneficial for cognitive impairment, by preventing the reduction of ID1 expression which may be associated with cognitive impairment.
Keywords: muscarinic acetylcholine receptor, cholinergic transmission, cognitive deficits, DNA binding protein inhibitor, 4-hydroxy-3-methoxybenzaldehyde
Introduction
The hippocampus is an important component of the limbic system and serves a role in regulating emotionality and cognitive processes, including memory consolidation, learning, and information retrieval (1–4). The loss of cholinergic function in the hippocampus is correlated with marked cognitive impairment (5,6).
Scopolamine, a muscarinic acetylcholine receptor antagonist, interferes with cholinergic transmission in the brain (7). It has been reported that scopolamine may impair memory performance in humans and animals (8) and that scopolamine may induce dysregulation of cholinergic activity in the hippocampus, leading to impairments in learning and memory (9,10). Therefore, scopolamine has been used for the induction of learning and memory impairment in experimental animals (11–13).
4-Hydroxy-3-methoxybenzaldehyde (vanillin) is a phenolic constituent synthesized by various types of plants, including Gastrodia elata Blume (Orchidaceae) (14,15). Previous studies have suggested that vanillin has a variety of beneficial effects against brain injuries via a number of therapeutic properties, including anti-inflammatory, antioxidant and anti-cancer activities (16–19). For example, vanillin administered following cerebral ischemia prevented neuronal damage/death in the CA1 area of the hippocampus in gerbils (20). However, few studies regarding effects of vanillin against scopolamine-induced cognitive impairment have been reported.
DNA binding protein inhibitor (ID) proteins control gene transcription via inhibitory binding to basic helix-loop-helix (bHLH) transcription factors, and four members of this protein family (ID1, 2, 3 and 4) have been identified in mammals (21–24). Members of the ID protein family share a highly conserved bHLH domain and are similar in size (13–20 kDa); however, these proteins display extensive sequence variation outside the bHLH domain (25). ID proteins, as transcription factors, serve roles in cell cycle regulation and apoptosis, the development of the nervous system, muscle development and tumorigenesis (24,26,27). It has been reported that ID1-imunoreactive cells are γ-aminobutyric acid (GABA) ergic interneurons in the gerbil hippocampus (28), and that GABAergic neuronal dysfunction is responsible for network alteration associated with cognitive deficits in Alzheimer's disease and aging (29–31). To the best of our knowledge, however, few studies regarding alterations in ID1 expression in animal models of scopolamine-induced cognitive deficits have been reported. Therefore, the present study examined the long-term effects of treatment with vanillin on scopolamine-induced cognitive impairment and alterations in ID1 expression in the mouse hippocampus.
Materials and methods
Experimental animals
A total of 210 male ICR mice (25–30 g body weight; 8 weeks of age) were used, and were handled according to the guidelines of the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011). The present study was approved based on ethical procedures and scientific care by the Kangwon National University Institutional Animal Care and Use Committee (approval no. KW-130424-2). The mice were maintained under a 12 h light/dark cycle at 23°C and 60% humidity with free access to food and water.
Animals (n=70/group) were intraperitoneally injected with 1 mg/kg scopolamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) once daily for 4 weeks. The scopolamine dose was selected based on previously published studies (32,33). Scopolamine-treated animals were simultaneously administered 40 mg/kg vanillin (Sigma-Aldrich; Merck KGaA), which was suspended in 1 ml 10% Tween-80 solution. The vanillin dose was selected based on a previous study (20) and was orally administered using a feeding needle once daily for 4 weeks. Vehicle -treated control mice were treated with the same volume of saline. Mice were sacrificed at 1, 2, 3 and 4 weeks following treatment with saline or scopolamine + vanillin (n=14 at each time point). The mice were weighed twice per week; no significant differences were observed in body weight between all the groups (data not shown).
Passive avoidance test
Short-term memory capacity was determined by assessing the latency in a passive avoidance test, according to the method of Horisawa et al (34). The test was performed with an apparatus consisting of light and dark compartments with a grid floor (GEM 392; San Diego Instruments, Inc., San Diego, CA, USA). In the training session, mice were permitted to explore the environments of the two compartments for 1 min, and the mice were administered an inescapable foot shock (0.3 mA for 3 sec) upon entering the dark compartment. The test session was performed 15 min subsequent to the training session, without applying the foot shock. The latency time of the passive avoidance test was defined as the difference between the start of the test session and the entry of the mouse into the dark compartment. Latency was recorded as 180 sec when the mouse did not enter the dark compartment within 180 sec.
Water maze performance
Spatial learning and memory were tested using the Morris water maze task, using the procedure of Wang et al (35). A circular pool of 90 cm in diameter and 45 cm in height was filled with water and divided into 4 sectors. A platform of 6 cm in diameter and 29 cm in height was placed in one sector, 1 cm below the surface of the water. Training was performed for 3 consecutive days prior to the test. The test was performed on the last day of each week. Mice were permitted to swim for 120 sec to search for the hidden platform. At the end of each trial, each mouse remained on the platform for 3 sec. The escape latency, the time taken to find the platform, was recorded using the Noldus Ethovision video tracking system (Ethovision XT; Noldus Information Technology B.V., Wageningen, The Netherlands).
Western blot analysis
ID1 levels in the mouse hippocampus (n=7 at each time point) were analyzed using a previously-published method (28). Hippocampal tissues were homogenized and ID1 levels were determined using a micro bicinchoninic acid (BCA) protein assay kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Nitrocellulose transfer membranes (Pall Life Sciences, Port Washington, NY, USA) were incubated with rabbit anti-ID1 (1:1,000; sc-488; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), β-actin (1:2,000, sc-47778; Santa Cruz Biotechnology, Inc.) and exposed to peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:2,000; sc-2004; Santa Cruz Biotechnology, Inc.) for 2 h at 4°C and an enhanced luminol-based chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.). The results of this analysis were scanned and densitometric analysis, as the relative optical density (ROD), was used for quantification of the bands using Scion Image software, version 2.0 (Scion Corporation, Frederick, MD, USA). A ratio of the ROD was calibrated as a percentage, with control mice designated as 100%.
Tissue processing for histology
In short, as previously described (30), mice were anaesthetized (n=7 at each time point) with sodium pentobarbital (30 mg/kg; intraperitoneal injection) and tissues were fixed with 4% paraformaldehyde for 6 h at 4°C. The brains were serially sectioned into 30-µm coronal sections using a cryostat (Leica Microsystem GmbH, Wetzlar, Germany).
Fluoro-Jade B (F-J B) histofluorescence
In order to observe the localization of neuronal degeneration, F-J B histofluorescence staining was performed, according to a previously-published procedure (36). The sections were immersed in a 0.06% potassium permanganate solution and stained with 0.0004% F-J B (Histo-Chem, Inc., Jefferson, AR, USA) staining solution. The stained tissues were analyzed using an epifluorescent microscope (magnification, ×200; Zeiss AG, Oberkochen, Germany) with blue (450–490 nm) excitation light and a barrier filter.
Immunohistochemistry
Immunohistochemistry for NeuN (a neuronal marker) and ID1 was performed using a published procedure (28). The sections were incubated in mouse anti-NeuN (1:800; MAB377; EMD Millipore, Billerica, MA, USA) or rabbit anti-ID1 (1:200; sc-488; Santa Cruz Biotechnology, Inc.) as primary antibodies, and in biotinylated horse anti-mouse (1:200; BA-2001; Vector Laboratories, Inc., Burlingame, CA, USA or rabbit anti-goat immunoglobulin G (1:200; BA-1100; Vector Laboratories, Inc.) and streptavidin peroxidase complex (VECTASTAIN® Elite ABC kit 1:200; Vector Laboratories, Inc.) as secondary antibodies. The antibodies were finally visualized with 3,3′-diaminobenzidine tetrahydrochloride. A negative control test was performed to establish the specificity of the immunostaining using pre-blocking serum (S-1000; Vector Laboratories, Inc.) instead of primary antibody. The negative control test showed no immunoreactivity in structures observed.
Data analysis
As previously described (37), the numbers of NeuN-immunoreactive and F-J B-positive cells were counted. A total of 10 sections per mouse were selected according to anatomical landmarks (−1.4 to −2.2 mm from anterior to posterior) of the gerbil brain atlas. Cells were counted in a 200×200-µm square at the center of the stratum pyramidale of the CA1-3 and dentate gyrus by averaging total cell numbers from each mouse per group. A ratio of the count was calibrated as a percentage of the sham group (NeuN-immunoreactive cells) or ischemia group (F-J B-positive cells).
Quantitative analysis of ID1 immunoreactivity was performed as previously described (37). In short, seven sections per animal were selected. Digital images of ID1-immunoreactive structures were captured with an AxioM1 light microscope (Zeiss AG) equipped with a digital camera (Axiocam; Zeiss AG) connected to a PC monitor, and the density of the structures was evaluated based on optical density (OD), which was obtained following the transformation of the mean gray level using the formula: OD=log (256/mean gray level). Background density in the images was subtracted, and brightness and contrast were calibrated as a percentage (ROD) using Adobe Photoshop version 8.0 (Adobe Systems, Inc., San Jose, CA, USA) and analyzed using ImageJ software, version 1.59 (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as a percentage, with control mice designated as 100%.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. The test of normality was performed using the Kolmogorov and Smirnov test for assessing normal distributions, and the Bartlett test for assessing identical standard distributions. All data passed the normality test. A multiple-sample comparison was applied to test the differences between groups (analysis of variance and the Tukey multiple range test as post hoc test using the criterion of the least significant differences). Statistical analysis was performed using GraphPad Prism, version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Passive avoidance test
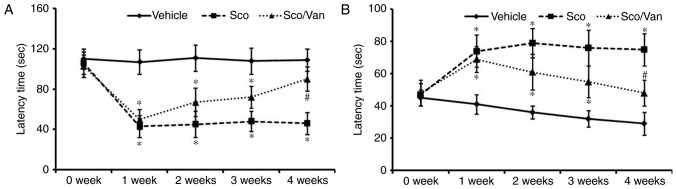
The passive avoidance test was performed to examine the protective effect of vanillin against scopolamine-induced learning and memory defects (Fig. 1A). No significant difference was observed in the latency time over the 4 weeks in the vehicle-treated mice. However, a reduction in the latency time was observed in the scopolamine-treated mice; the latency time was significantly decreased at 1 week post-treatment with scopolamine, and the decreased latency time was maintained until 4 weeks (Fig. 1A). Cotreatment with scopolamine and vanillin improved the reduction of the latency time from 2 weeks following the cotreatment, and the decreased latency times was significantly recovered 4 weeks subsequent to the cotreatment (Fig. 1A).
Figure 1.
Effects of vanillin against the scopolamine-induced alteration of cognitive defects. (A) Passive avoidance test. (B) Morris water maze test. n=14 mice/group. *P<0.05 vs. vehicle; #P<0.05 vs. Sco. Error bars indicate the mean ± standard error of the mean. Sco, scopolamine-treated; Sco/Van, scopolamine/vanillin-cotreated.
Morris water maze test
The Morris water maze test was performed to investigate the effect of vanillin against scopolamine-induced spatial memory impairment. As presented in Fig. 1B, the vehicle-treated mice readily learned and memorized the position of the submerged hidden platform, and exhibited significantly decreased escape latency at 4 weeks post-training. However, in the scopolamine-treated mice, the escape latency was significantly longer compared with the vehicle-treated mice at 1 week following treatment with scopolamine, and the increased escape latency was maintained until 4 weeks (Fig. 1B). Cotreatment with scopolamine and vanillin improved the escape latency from 2 weeks following the cotreatment, and significantly recovered the escape latency at 4 weeks subsequent to the cotreatment (Fig. 1B).
ID-1 protein levels
According to the results of the western blot analysis, the level of ID-1 protein in the scopolamine-treated mice gradually decreased from 1 week following treatment with scopolamine compared with the vehicle-treated mice, and was significantly decreased at 2 and 4 weeks post-treatment with scopolamine (Fig. 2). However, cotreatment with scopolamine and vanillin increased the ID-1 protein level to a level consistent with that in the vehicle-treated mice at 4 weeks subsequent to the cotreatment (Fig. 2).
Figure 2.
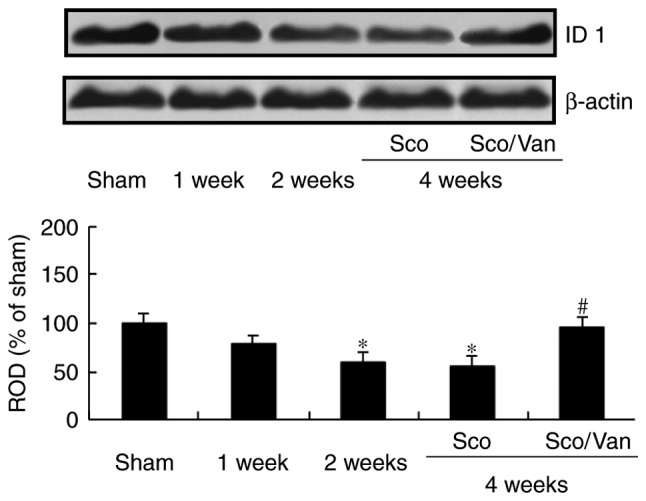
Western blot analysis of ID1 in the hippocampus. Cotreatment with scopolamine and vanillin restored the ID1 level at 4 weeks following cotreatment. ROD as percentage values of the immunoblot band is presented. n=7 mice/group. *P<0.05 vs. vehicle; #P<0.05 vs. Sco at 4 weeks. Error bars indicate the mean ± standard error of the mean. Sco, scopolamine-treated; Sco/Van, scopolamine/vanillin-cotreated; ID1, DNA binding protein inhibitor ID-1; ROD, relative optical density.
Neuronal damage
Neuronal damage following treatment with scopolamine was examined in the hippocampus via NeuN immunohistochemistry and F-J B histofluorescence staining (data not shown). NeuN-immunoreactive cells were observed in the pyramidal layer of the hippocampus (CA1-3 areas) and in the granule cell layer of the dentate gyrus of the vehicle-treated mice. The morphology of NeuN-immunoreactive cells in the scopolamine-treated mice was consistent with that in the vehicle-treated mice. In addition, F-J B-positive cells, which are damaged cells, were not observed in any regions of the scopolamine-treated mice. This result was consistent with the results of previous studies (10,33). These findings indicated that treatment with scopolamine did not evoke neuronal death in the hippocampus.
ID-1 immunoreactivity
CA1 area
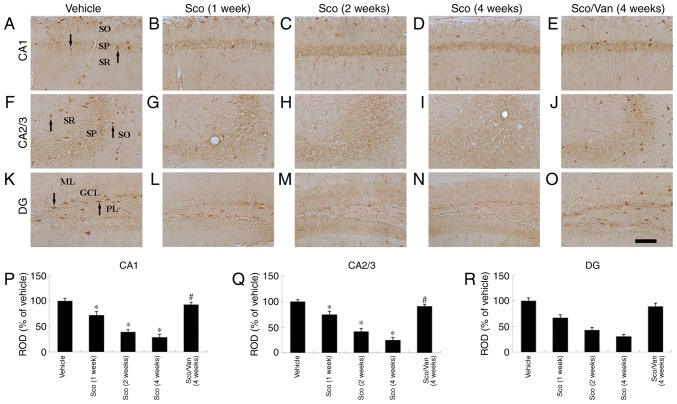
ID-1 immunohistochemistry results are presented in Fig. 3. ID-1-immunoreactive cells were distributed in all layers of the CA1 area in the vehicle-treated mice (Fig. 3A). In the scopolamine-treated mice, ID-1 immunoreactivity was gradually decreased from 1 week following treatment with scopolamine, and ID-1 immunoreactivity was markedly decreased at 4 weeks post-treatment with scopolamine (Fig. 3B-D). However, the distribution pattern of ID-1-immunoreactive structures in the scopolamine and vanillin-cotreated mice was similar to that in the vehicle-treated mice (Fig. 3E). In addition, the ROD of ID-1 immunoreactivity in the hippocampal areas are presented in Fig. 3P-R.
Figure 3.
ID1 immunohistochemistry in the CA1, CA2/3 and dentate gyrus (DG) regions of the vehicle-treated (A, F, K), scopolamine-treated (B-D, G-I, L-N), and scopolamine/vanillin-treated mice (E, J, O). ID1-immunoreactive cells (arrows) in the vehicle-treated mice were observed in CA1-3 and the dentate gyrus. In the scopolamine-treated mice, ID1-immunoreactive cells were markedly decreased from 1 week post-treatment with scopolamine. In the cotreated mice, ID1-immunoreactive cells were markedly increased compared with the vehicle-treated mice. Scale bar, 100 µm. (P, Q and R) ROD as percentage values of ID1 immunoreactivity in the mouse hippocampus. n=7 mice/group. *P<0.05 vs. vehicle; #P<0.05 vs. Sco at 4 weeks. Error bars indicate the mean ± standard error of the mean. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; Sco, scopolamine-treated; Sco/Van, scopolamine/vanillin-cotreated; ID1, DNA binding protein inhibitor ID-1; ROD, relative optical density.
CA2/3 area
The pattern of ID-1-immunoreactive structures in the CA2/3 area following treatment with scopolamine was broadly similar to the alteration in ID-1-immunoreactivity in the CA1 area (Fig. 3F-I). Cotreatment with scopolamine and vanillin generated results similar to those in the CA1 area (Fig. 3J).
Dentate gyrus
ID-1-immunoreactive cells were primarily distributed in the polymorphic layer in the vehicle-treated mice (Fig. 3K). At 1 week post-treatment with scopolamine, ID-1 immunoreactive cells and immunoreactivity were markedly decreased (Fig. 3L). Thereafter, ID-1 immunoreactivity was gradually decreased following scopolamine treatment (Fig. 3M and N). However, ID-1-immunoreactivity in the co-treated mice was similar to that in the vehicle-treated mice at 4 weeks post-cotreatment (Fig. 3O).
Discussion
The passive avoidance test is a measurement of learning and memory based on avoidance of a fear-inducing stimulus. In the passive avoidance test, rodents naturally prefer dark compartments; however, receiving an electric shock in the dark compartment causes a conflict to this tendency (38). The Morris water maze test is performed to assess spatial memory in rodents (39,40). In the present study, chronic systemic treatment with scopolamine induced an impairment in learning and memory, and spatial memory, in mice from 1 week post-treatment, and the impairment deteriorated over time, as observed in the passive avoidance and water maze tests. Based on the above previous reports and the results of the present study, chronic systemic treatment with scopolamine may be a viable method for developing cognitive impairment in rodents.
The results of the present study demonstrated that no neuronal loss was present in the mouse hippocampus following treatment with scopolamine for 4 weeks via NeuN immunohistochemistry and F-J B histofluorescence, proven histological methods for the examination of neuronal death or loss in the brain. Recently, it was reported that chronic treatment with scopolamine induced a significant reduction in neurogenesis of the hippocampal dentate gyrus in mice, closely associated with hippocampus-dependent learning and memory, without loss of neurons in the mouse dentate gyrus (33). This previous result indicated that chronic systemic treatment with scopolamine may induce cognitive deficits without neuronal loss in the mouse hippocampus.
The present study evaluated the effects of vanillin on scopolamine-induced cognitive impairment using passive avoidance and the Morris water maze tests in mice, and revealed that treatment with vanillin significantly attenuated cognitive impairment induced by scopolamine. Vanillin has been demonstrated to display multifunctional effects, including anti-inflammatory, antioxidant and anti-cancer effects (16–19). Recently, Gupta and Sharma (41) demonstrated that vanillin markedly attenuated learning and memory deficits in a rat model of Huntington's disease induced by 3-nitropropionic acid. It is known that scopolamine causes cognitive impairments by decreasing central cholinergic activity in experimental animals (42–44). In addition, Shi et al (45) reported that long-term scopolamine injections led to cognitive deficits, closely associated with cyclic-AMP responsive element-binding protein signaling activity in the cerebral cortex and dorsal hippocampus in rats.
Recently, it was reported that vanillin and 4-hydroxybenzyl alcohol effectively attenuated learning and memory impairment, and the reduction of cell proliferation and neuroblast differentiation, in the mouse hippocampal dentate gyrus following treatment with scopolamine (46). This previous result indicated that vanillin may reverse the scopolamine-induced decrease in cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus. In this regard, adult neurogenesis in the hippocampus is implicated in learning and memory processes (47). ID proteins are included in a number of physiological and pathological processes, including cell growth, differentiation, senescence and survival (24,26,27). In particular, ID proteins serve important roles in adult neurogenesis through the regulation of quiescence and self-renewal of neural stem cells, in addition to neuronal differentiation (48–51). For example, ID1 exhibits precocious neurogenesis by sustaining transcription factor HES-1 expression in the mouse brain (52). To the best of our knowledge, no studies regarding the association between ID proteins and scopolamine-induced cognitive deficits have been reported, and the alteration of ID expression in the hippocampus with cognitive impairment has not investigated. The present study examined whether vanillin may improve scopolamine-induced cognitive impairment by preventing the reduction of immunoreactivity and the expression of ID1 protein in the mouse hippocampus, and it was observed that the ID1 protein level in the hippocampus was decreased from 1 week and was significantly decreased at 4 weeks post-treatment with scopolamine. In addition, ID1 immunoreactivity in cells in the hippocampal CA1-3 areas and dentate gyrus significantly decreased from 1 week and disappeared 4 weeks post-treatment with scopolamine. Therefore, it is likely that a decrease in or inhibition of ID1 expression in hippocampal neurons following chronic systemic treatment with scopolamine may be associated with scopolamine-induced cognitive deficits, and that treatment with vanillin in scopolamine-treated mice significantly restored ID1-immunoreactive cells and expression 4 weeks subsequent to the treatment.
GABAergic interneurons synthesize and release GABA, and contribute substantially to inhibitory regulation in the adult neuronal network (53). It was previously demonstrated that cognitive deficiency is caused by the degeneration of GABAergic neurons in the medial septal region (54,55). Studies have reported that GABAergic neuronal dysfunction is responsible for the network alteration associated with cognitive deficits in Alzheimer's disease and aging (29–31). In addition, GABAergic neurons express the muscarinic acetylcholine receptor (56), and scopolamine blocks muscarinic receptors in GABAergic neurons. Studies have suggested that the muscarinic acetylcholine receptor may mediate the antidepressant action of scopolamine (57,58). According to these previous results, it may be suggested that GABAergic-cholinergic interactions in the hippocampus may affect cognitive deficits. Li et al (59) reported that GABAergic interneuronal dysfunction may lead to an impairment in adult hippocampal neurogenesis in mice. In addition, a recent study reported that ID1-immunoreactive cells were identified as GABAergic interneurons (28). In the present study, ID1 immunoreactivity, which is expressed in GABAergic interneurons in the mouse hippocampus, was readily inhibited by chronic treatment with scopolamine. Based on the above findings, it may be suggested that the ID1 protein is involved in the dysfunction of GABAergic neurons following treatment with scopolamine.
In conclusion, the results of the present study revealed that vanillin was able to significantly improve scopolamine-induced cognitive impairment by preventing the reduction of immunoreactivity and expression of ID1 protein in the mouse hippocampus. Therefore, vanillin may be efficacious for the prevention and treatment of cognitive impairment and is a worthy candidate for clinical evaluation.
Acknowledgements
The present study was supported by the Bio-Synergy Research Project (grant no. NRF-2015M3A9C4076322) of the Ministry of Science, ICT and Future Planning through the National Research Foundation and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (grant no. 2017R1A2B4008403).
References
- 1.Hami J, Kheradmand H, Haghir H. Sex differences and laterality of insulin receptor distribution in developing rat hippocampus: An immunohistochemical study. J Mol Neurosci. 2014;54:100–108. doi: 10.1007/s12031-014-0255-1. [DOI] [PubMed] [Google Scholar]
- 2.Huang W, Cao J, Liu X, Meng F, Li M, Chen B, Zhang J. AMPK Plays a dual role in regulation of CREB/BDNF pathway in mouse primary hippocampal cells. J Mol Neurosci. 2015;56:782–788. doi: 10.1007/s12031-015-0500-2. [DOI] [PubMed] [Google Scholar]
- 3.Kong Y, Bai PS, Sun H, Nan KJ. Expression of the newly identified gene CAC1 in the hippocampus of Alzheimer's disease patients. J Mol Neurosci. 2012;47:207–218. doi: 10.1007/s12031-012-9717-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Sun C, Xiong L, Yang Y, Gao Y, Wang L, Zuo H, Xu X, Dong J, Zhou H, Peng R. MicroRNAs: Novel mechanism involved in the pathogenesis of microwave exposure on Rats' Hippocampus. J Mol Neurosci. 2014;53:222–230. doi: 10.1007/s12031-014-0289-4. [DOI] [PubMed] [Google Scholar]
- 5.Lippa AS, Critchett DJ, Ehlert F, Yamamura HI, Enna SJ, Bartus RT. Age-related alterations in neurotransmitter receptors: An electrophysiological and biochemical analysis. Neurobiol Aging. 1981;2:3–8. doi: 10.1016/0197-4580(81)90052-X. [DOI] [PubMed] [Google Scholar]
- 6.Vijayan VK. Cholinergic enzymes in the cerebellum and the hippocampus of the senescent mouse. Exp Gerontol. 1977;12:7–11. doi: 10.1016/0531-5565(77)90026-2. [DOI] [PubMed] [Google Scholar]
- 7.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 8.Grasby PM, Frith CD, Paulesu E, Friston KJ, Frackowiak RS, Dolan RJ. The effect of the muscarinic antagonist scopolamine on regional cerebral blood flow during the performance of a memory task. Exp Brain Res. 1995;104:337–348. doi: 10.1007/BF00242019. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Sur B, Shim J, Hahm DH, Lee H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2014;14:338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo DY, Choi JH, Kim W, Nam SM, Jung HY, Kim JH, Won MH, Yoon YS, Hwang IK. Effects of luteolin on spatial memory, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia model. Neurol Res. 2013;35:813–820. doi: 10.1179/1743132813Y.0000000217. [DOI] [PubMed] [Google Scholar]
- 11.Ebert U, Kirch W. Scopolamine model of dementia: Electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 12.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, You Z, Wu Z, Zhou L, Shen J, Gu Z. WY14643 attenuates the scopolamine-induced memory impairments in mice. Neurochem Res. 2016;41:2868–2879. doi: 10.1007/s11064-016-2002-1. [DOI] [PubMed] [Google Scholar]
- 14.Jung JW, Yoon BH, Oh HR, Ahn JH, Kim SY, Park SY, Ryu JH. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol Pharm Bull. 2006;29:261–265. doi: 10.1248/bpb.29.261. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Ha JH, Yong CS, Lee DU, Huh K, Kang YS, Lee SH, Jung MW, Kim JA. Inhibitory effects of constituents of Gastrodia elata Bl. On glutamate-induced apoptosis in IMR-32 human neuroblastoma cells. Arch Pharm Res. 1999;22:404–409. doi: 10.1007/BF02979066. [DOI] [PubMed] [Google Scholar]
- 16.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Murakami Y, Hirata A, Ito S, Shoji M, Tanaka S, Yasui T, Machino M, Fujisawa S. Re-evaluation of cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in macrophages stimulated with lipopolysaccharide. Anticancer Res. 2007;27:801–807. [PubMed] [Google Scholar]
- 18.Wu SL, Chen JC, Li CC, Lo HY, Ho TY, Hsiang CY. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J Pharmacol Exp Ther. 2009;330:370–376. doi: 10.1124/jpet.109.152835. [DOI] [PubMed] [Google Scholar]
- 19.Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Hwang IK, Won MH. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007;1181:130–141. doi: 10.1016/j.brainres.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 21.Kee Y, Bronner-Fraser M. To proliferate or to die: Role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes Dev. 2005;19:744–755. doi: 10.1101/gad.1257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari ME, Murre C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/MCB.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- 24.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/S0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 25.Nair R, Teo WS, Mittal V, Swarbrick A. ID proteins regulate diverse aspects of cancer progression and provide novel therapeutic opportunities. Mol Ther. 2014;22:1407–1415. doi: 10.1038/mt.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasorella A, Benezra R, Iavarone A. The ID proteins: Master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 27.Ling F, Kang B, Sun XH. Id proteins: Small molecules, mighty regulators. Curr Top Dev Biol. 2014;110:189–216. doi: 10.1016/B978-0-12-405943-6.00005-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee JC, Chen BH, Cho JH, Kim IH, Ahn JH, Park JH, Tae HJ, Cho GS, Yan BC, Kim DW, et al. Changes in the expression of DNA-binding/differentiation protein inhibitors in neurons and glial cells of the gerbil hippocampus following transient global cerebral ischemia. Mol Med Rep. 2015;11:2477–2485. doi: 10.3892/mmr.2014.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, Ring K, Zwilling D, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS One. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: The role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Wang ZH, Wu YY, Tang H, Tan L, Wang X, Gao XY, Xiong YS, Liu D, Wang JZ, Zhu LQ. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol Neurobiol. 2013;47:373–381. doi: 10.1007/s12035-012-8355-9. [DOI] [PubMed] [Google Scholar]
- 33.Yan BC, Park JH, Chen BH, Cho JH, Kim IH, Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, et al. Long-term administration of scopolamine interferes with nerve cell proliferation, differentiation and migration in adult mouse hippocampal dentate gyrus, but it does not induce cell death. Neural Regen Res. 2014;9:1731–1739. doi: 10.4103/1673-5374.143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horisawa T, Ishibashi T, Nishikawa H, Enomoto T, Toma S, Ishiyama T, Taiji M. The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: Mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res. 2011;220:83–90. doi: 10.1016/j.bbr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Li J, Wang Z, Xue L, Zhang Y, Chen Y, Su J, Li Z. L-tyrosine improves neuroendocrine function in a mouse model of chronic stress. Neural Regen Res. 2012;7:1413–1419. doi: 10.3969/j.issn.1673-5374.2012.18.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candelario-Jalil E, Alvarez D, Merino N, León OS. Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res. 2003;47:245–253. doi: 10.1016/S0168-0102(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 37.Lee JC, Kim IH, Cho GS, Park JH, Ahn JH, Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, et al. Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. J Neurol Sci. 2014;336:74–82. doi: 10.1016/j.jns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: Corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 39.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 40.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Sharma B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington's disease. Pharmacol Biochem Behav. 2014;122:122–135. doi: 10.1016/j.pbb.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Blake MG, Boccia MM, Krawczyk MC, Baratti CM. Scopolamine prevents retrograde memory interference between two different learning tasks. Physiol Behav. 2011;102:332–337. doi: 10.1016/j.physbeh.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Souza AC, Bruning CA, Acker CI, Neto JS, Nogueira CW. 2-Phenylethynyl-butyltellurium enhances learning and memory impaired by scopolamine in mice. Behav Pharmacol. 2013;24:249–254. doi: 10.1097/FBP.0b013e32836353a5. [DOI] [PubMed] [Google Scholar]
- 44.Ohno M, Watanabe S. Interactive processing between glutamatergic and cholinergic systems involved in inhibitory avoidance learning of rats. Eur J Pharmacol. 1996;312:145–147. doi: 10.1016/0014-2999(96)00580-8. [DOI] [PubMed] [Google Scholar]
- 45.Shi Z, Chen L, Li S, Chen S, Sun X, Sun L, Li Y, Zeng J, He Y, Liu X. Chronic scopolamine-injection-induced cognitive deficit on reward-directed instrumental learning in rat is associated with CREB signaling activity in the cerebral cortex and dorsal hippocampus. Psychopharmacology (Berl) 2013;230:245–260. doi: 10.1007/s00213-013-3149-y. [DOI] [PubMed] [Google Scholar]
- 46.Kim YH, Park JH. Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia. Anat Cell Biol. 2017;50:143–151. doi: 10.5115/acb.2017.50.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Jen Y, Manova K, Benezra R. Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev Dyn. 1997;208:92–106. doi: 10.1002/(SICI)1097-0177(199701)208:1<92::AID-AJA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Jung S, Park RH, Kim S, Jeon YJ, Ham DS, Jung MY, Kim SS, Lee YD, Park CH, Suh-Kim H. Id proteins facilitate self-renewal and proliferation of neural stem cells. Stem Cells Dev. 2010;19:831–841. doi: 10.1089/scd.2009.0093. [DOI] [PubMed] [Google Scholar]
- 50.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 51.Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai G, Sheng N, Xie Z, Bian W, Yokota Y, Benezra R, Kageyama R, Guillemot F, Jing N. Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell. 2007;13:283–297. doi: 10.1016/j.devcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 54.Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus. 2011;21:835–846. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roland JJ, Stewart AL, Janke KL, Gielow MR, Kostek JA, Savage LM, Servatius RJ, Pang KC. Medial septum-diagonal band of Broca (MSDB) GABAergic regulation of hippocampal acetylcholine efflux is dependent on cognitive demands. J Neurosci. 2014;34:506–514. doi: 10.1523/JNEUROSCI.2352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van der Zee EA, Luiten PG. GABAergic neurons of the rat dorsal hippocampus express muscarinic acetylcholine receptors. Brain Res Bull. 1993;32:601–609. doi: 10.1016/0361-9230(93)90161-4. [DOI] [PubMed] [Google Scholar]
- 57.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, Dwyer JM, Fuchikami M, Becker A, Drago F, Duman RS. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]