Abstract
This study was performed in order to investigate possible role of brain β-endorphins as markers of antidepressive drugs therapy monitoring. Experiment was done using amitriptyline and trazodone as antidepressants. For quantification of brain β-endorphins we used RIA technique. Our results showed significant decrease of brain β-endorphins concentration in drug-pretreated animals, vs. those in of control group treated with 0,95% NaCl. The lower values were obtained in trazodone pre-treated animals. This study shows that use of psychoactive drugs have influence on brain β-endorphins concentration. β-endorphins could be of great importance, used as markers for evaluation of patient treatment.
Keywords: amitriptyline, trazodone, β-endorphins, rat, brain
INTRODUCTION
The investigations of relation between endorphins and depression began with findings of enkephalin and opioid receptors located in mood-response areas of the brain. Previous research raised a question whether excess, deficiency, or static levels of endorphins cause depression. Also, it was hypothesized that endorphins may not be a factor in depression at all. Therefore, it was presumed that it is premature to conclude how the endogenous opioid system is involved in depression. Endorphins are likely to modulate the nervous system activity over the long-term rather than short time period. Endorphins are released in shock, freeze, “fight or flight”, trauma, physical pain and in all stress including psychological stress. They serve as an analgesic (pain killing), anesthetic and cause dissociation, immobilization and loss of self. Depressives induce elevation of stress hormone levels in blood. Since endorphins are released along with ACTH in response to any stressor, depressives are supposed to “elevate” endorphin levels as well (1,2).
ANTIDEPRESSANT DRUGS
There are numerous antidepressant medications, which are specifically designed to mimic the effects of endorphins in the brain to make a depressed person feel better or cope with stressful situations. Because of stimulated production of endorphins, studies have shown that physical activity can have a similar effect as antidepressants. But unlike antidepressants, there are no negative physiological side effects of exercise. Antidepressants can also take between two and three weeks before showing the improvement of effects, whereas exercise can boost your mood instantly. Investigations in the field of psychopharmaceutical drugs gave us a lot of knowledge about their positive and side effects. Tricycle antidepressants were first in use, expressing their effects as a supplement for primary role - inhibition of norepinephrin and serotonin uptake by the nervous ends (1, 2). More recently, practice patterns have shifted to the antidepressant class as the most commonly prescribed for anxiety disorders. This is due to their proven efficacy in anxiety as well as in commonly co-morbid depressive symptoms. Although efficacy between the selective serotonin reuptake inhibitors (SSRIs) and the tricyclic antidepressants (TCAs) is similar, the SSRIs have an enhanced safety and tolerability profile, making them the preferred agent for all anxiety disorders (3). The efficacy of each antidepressant available has been found equal to that of amitriptyline in double-blind studies as far as mild to moderate depression is involved. However, it seems that some antidepressants are more effective than others in the treatment of severe types of depression (4). The considerable side effect burden, the possibility of producing anticholinergic delirium, and their contribution to promoting falls and hip fractures, make TCAs especially unsuitable for the elderly and no longer recommended. SSRIs have been proved to relieve many of the anxiety and/or depressive symptoms associated with anxiety disorders, such as generalized anxiety disorder (GAD), social anxiety disorder (SAD), panic disorder, obsessive-compulsive disorder (OCD), and post-traumatic stress disorder (PTSD). They are effective, well tolerated, and relatively safe in overdose situations, making them an ideal choice for the treatment of anxiety, particularly for the elderly population. Although the majority of SSRIs have gained US Food and Drug Administration (FDA) approval for major depressive disorder, they may not be interchangeable in the treatment of anxiety disorders, because each SSRI has different FDA approvals for the anxiety disorders (3). Trazodone, nefazodon and bupropion has less defined neuropharmacology and are taken as atipic. Nevertheless, they have better efficiency and endurance, leading to better acceptance. Trazodone is an effective antidepressant drug with a broad therapeutic spectrum, including anxiolytic efficacy. This tri-azolopyridine antidepressant, is currently the second most commonly prescribed agent for the treatment of insomnia due to its sedating qualities. Given trazodone’s widespread use, a careful review of the literature was conducted to assess its efficacy and side effects when given for treatment of insomnia. Although trazodone is usually referred to as a serotonin (5-HT) reuptake inhibitor, this pharmacological effect appears to be too weak to fully account for its clinical effectiveness (5, 6).
FIGURE 1.

Structure of amitriptyline
FIGURE 2.

Trazodone structure from (fromwww.3dchem.com/imagesofinolecules/)
ANTIDEPRESSANTS AND Β-ENDORPHINS
Previous investigations showed that acute amitriptyline and clomipramine produce naloxone-reversible antino-ciception. This apparent opioid-like involvement was further investigated by measuring β-endorphin levels in the hypothalamus following acute and chronic treatment with these antidepressants. They demonstrated significantly raised levels of β-endorphin. The support was provided for the suggestion that antidepressants activate opioid systems, through both a direct opioid receptor interaction and an indirect action through enhanced release of opioid peptides. Moreover, it is postulated that the direct action of antidepressants on opioid receptors and the endogenous opioid peptides released interact as agonists at both μ- and δ-opioid receptors to inhibit nociceptive transmission, since the activity is antagonized by both naloxone and nal-trindole (7). Research showed synergistic influence of trazodone, combined with mud bath in therapy of fibro-myalgic syndrome, bettering psychological response of homeostasis formation and systems as an answer to stress. Desipramine and paroxetin, used in animal depression models did not significantly affect the extracellular levels of β-endorphins in nucleus accumbens, but chronic antidepressant treatment did normalize serotonin-induced release of β-endorphins, as well as behavioral manifestation of depressive behaviour (8, 9).
MATERIAL AND METHODS
Albino Wistar rats, weight 250 g were used, divided in groups of 6, with each animal control to itself. Ethical Committee of our Institution approved the experiment. Amitriptyline (2mg/kg/day) and trazodone (5mg/kg/day) were administrated to experimental, and 0,95% NaCl solution to control group. Before brain samples were collected, all animals were properly sacrificed. Collection of brain samples was performed immediately for control group, and after 1st and 9th day of amitriptyline and trazodone administration in treated animals. For analyzing β-endorphin levels we used RIA technique, for quantification of human serum and brain β-endorphin (Nichols Institute, San Juan, Capistrano, USA), and for radioactivity level β-counter with gamma-radiation source (LKB Wallac - Sweden). β-endorphin concentration is directly proportional to radioactivity measured in samples. Concentration is given in pg/g for brain β-endorphin values. Counting mean value, standard deviation and standard error we performed statistic evaluation of obtained results. The level of significance was determined by use of Student’s T test, with values p<0,05 considered as significant.
RESULTS AND DISCUSSION
Our data, presented by charts and graphics, show brain β-endorphins values after 1st and 9th day of antidepressive drug administration. Obtained values for each day were compared to the other, and to those of control group. Our results are different compared to results of other authors (7). After 1st day results show significant decrease of brain β-endorphins, which was present also in rat brains after 9th day of continuous anti-depressant administration. In general, amitriptyline produced greater response considering higher brain β-endorphins concentration compared to trazodone. There was significant difference between brain β-en-dorphins values of animals treated with trazodone compared with amitriptyline group after 1st day of treatment. There was no significant difference between rat brain β-endorphins in other compared groups/days, showing lower values of brain β-endorphins concentration on each day of continuous trazodone versus amitriptyline administration (chart 1). Individual values for each animal of experimental groups are presented in Graphic 1. Our results show mostly lower brain β-endorphins values of animals treated with trazodone. Those differences may be dependent to lower brain β-endorphins values before beginning of treatment. We consider that all of these changes can be caused by differences in mechanism of triazolopyridine (trazodone) and tricyclic antidepressant drugs action on brain β-en-dorphins content. That points at possible differences in synthesis intensity of β-endorphins, degradation of brain β-endorphins and possible releasing into blood stream, caused by constant antidepressive drugs administration.
CHART 1.

Brain ß-endorphins values in antidepressive drugs treated animals
GRAPHIC 1.
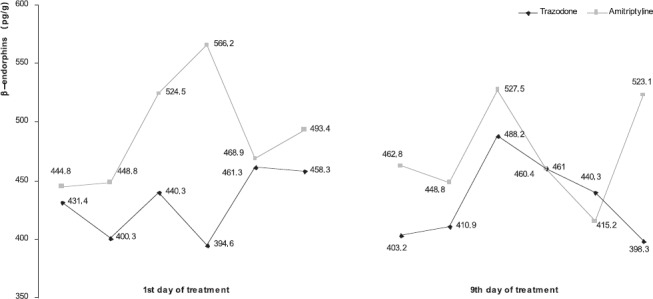
Individual values of brain ß-endorphins
CONCLUSION
- Rat brain β-endorphins values after a continuous antidepressive drugs treatment are significantly lower then those in control group
- In general, amitriptyline produced greater response considering higher brain β-endorphins concentration.
- Values of rat brain β-endorphins in trazodone pretreated animals are lower compared to values of amitripty-line treated groups
- Evaluation of brain β-endorphins level could be used as markers for investigation of psychoactive drug effects
REFERENCES
- 1.Gudman & Gilman’s. The pharmacological basis of therapeutics. Nintth edition. New York, St. Louis: McGraw-Hill: Health Professions Division; 1996. pp. 339–404. 432-433, 523. [Google Scholar]
- 2.Jadrić R, Zulić I, Hasić S, Kiseljaković E, Zečević B, Radovanović J, Ićinidić-Nakaš E, Winterhalter-Jadrić M. Trazodone influence on rat sera beta-endorphins level. Bosn J Basic Med. Sci. 2004;4(2):33–36. doi: 10.17305/bjbms.2004.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVane C.L, Chiao E, Franklin M, Kruep E.J. Anxiety disorders in the 21st century: status, challenges, opportunities, and comorbidity with depression. Am. J. Man. Care. 2005;11(12):344–353. [PubMed] [Google Scholar]
- 4.Schreiber S, Bleich A, Pick C.G. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects-a possible opioid involvement in severe depression? J. Mol. Neurosci. 2002;18(1-2):143–149. doi: 10.1385/JMN:18:1-2:143. [DOI] [PubMed] [Google Scholar]
- 5.Odagaki Y, Toyoshima R, Yamauchi T. Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by (35S) GTP gammaS binding. J. Psychopharmacol. 2005;19:235–241. doi: 10.1177/0269881105051526. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson W.B. A review of the evidence for the efficacy and safety of trazodone in insomnia. J. Clin. Psychiatry. 2005;66:469–476. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- 7.Gray A.M, Spencer P.S.J, Sewell R.D.E. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. British J Pharm. 1998;124:669–674. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellometi S, Galzigna L. Function of the hypothalamic adrenal axis in patients with fibromyalgia syndrome and undergoing mud-pack treatment. Int. J. Clin. Pharmacol. Res. 1999;19(1):27–33. [PubMed] [Google Scholar]
- 9.Zangen A, Nakash R, Roth-Deri I, Overstreet D.H, Yadid G. Impaired release of beta-endorphin in response to serotonin in a rat model of depression. Neuroscience. 2002;110(3):389–393. doi: 10.1016/s0306-4522(01)00612-1. [DOI] [PubMed] [Google Scholar]


