Abstract
New treatments for hepatitis C virus (HCV) may be highly effective but are associated with substantial costs that may compel clinicians and patients to consider delaying treatment. This study investigated the cost-effectiveness of these treatments with a focus on patients in early stages of liver disease. We developed a state-transition (or Markov) model to calculate costs incurred and quality-adjusted life-years (QALYs) gained following HCV treatment, and we computed incremental cost-effectiveness ratios (cost per QALY gained, in 2012 US dollars) for treatment at different stages of liver disease versus delaying treatment until the subsequent liver disease stage. Our analysis did not include the potential treatment benefits associated with reduced non–liver-related mortality or preventing HCV transmission. All parameter values, particularly treatment cost, were varied in sensitivity analyses. The base case scenario represented a 55-year-old patient with genotype 1 HCV infection with a treatment cost of $100,000 and treatment effectiveness of 90%. In this scenario, for a 55-year-old patient with moderate liver fibrosis (Metavir stage F2), the cost-effectiveness of immediately initiating treatment at F2 (versus delaying treatment until F3) was $37,300/QALY. For patients immediately treated at F0 (versus delaying treatment until F1), the threshold of treatment costs that yielded $50,000/QALY and $100,000/QALY cost-effectiveness ratios were $22,200 and $42,400, respectively.
Conclusion
Immediate treatment of HCV-infected patients with moderate and advanced fibrosis appears to be cost-effective, and immediate treatment of patients with minimal or no fibrosis can be cost-effective as well, particularly when lower treatment costs are assumed.
In the United States, hepatitis C virus (HCV) infection imposes a considerable burden of morbidity, mortality, and health care costs.1–4 An estimated 50% of US HCV infections remain undiagnosed,5–7 and of the total infected population as few as 38% of patients were referred to care.5 Among a sample of US veterans, only 12% of HCV-infected patients had initiated treatment.8 While the most recent guidelines recommend treatment for nearly all patients with chronic HCV infection, they recommend more “urgent initiation” of treatment among patients with advanced fibrosis or cirrhosis.9 In particular, patients with severe fibrosis (Metavir stage F3) and compensated cirrhosis (F4) are characterized as having the “highest” treatment priority and patients with moderate fibrosis (F2) are characterized as having “high” treatment priority.9 Liver fibrosis is a measure of apparent liver damage done by inflammation; the Metavir liver fibrosis scale ranges from F0 (no liver damage) to F4 (compensated cirrhosis). The key contribution of our study is to address these treatment guidelines by examining the decision to initiate treatment immediately or delay treatment until a patient progresses to a later stage of liver disease.
Effective and expensive treatment regimens present a challenge to payers and other stakeholders who must consider the costs and health benefits of HCV screening and treatment strategies. This study develops a cost-effectiveness model and utilizes disease-related and economically related parameters from published sources as well as from data obtained in the longitudinal Chronic Hepatitis Cohort Study (CHeCS).10 We calculated the costs and effects in quality-adjusted life-years (QALYs) gained following initiation of HCV treatment based on fibrosis level. We modeled treatment as a generic regimen of highly effective antivirals with a base case cost of $100,000 per patient. In sensitivity analyses we varied our assumptions about treatment cost, age, stage of liver fibrosis, and other parameters. Broadly, our objectives were to gain a better understanding of the relationships between treatment costs, cost-effectiveness, and stages of liver disease, as opposed to estimating the cost-effectiveness of a particular medical or pharmaceutical product. Consideration of treatment at early stages of liver disease is relevant to many of the approximately 3 million individuals who are infected with HCV.5 In a study of four large US medical centers, the majority of biopsied patients (62%) exhibited early stages of liver disease with fibrosis levels of F2, F1, or F0.11
Materials and Methods
Analytic Overview
We developed a cost-effectiveness model to represent the clinical experience of a diagnosed, chronically infected HCV patient (Fig. 1). Consistent with other studies,12–15 the model classifies patients according to treatment status and liver disease stage. The investigation compares a relatively constrained treatment strategy (treatment at fibrosis stages F3 and F4) to more expansive treatment strategies where treatment is initiated at earlier stages of liver disease (fibrosis stages F2, F1, or F0). The patient incurs medical costs and accrues QALYs annually. For a given treatment strategy, patients are treated only if their liver disease stage is at or beyond the fibrosis stage associated with the given strategy.
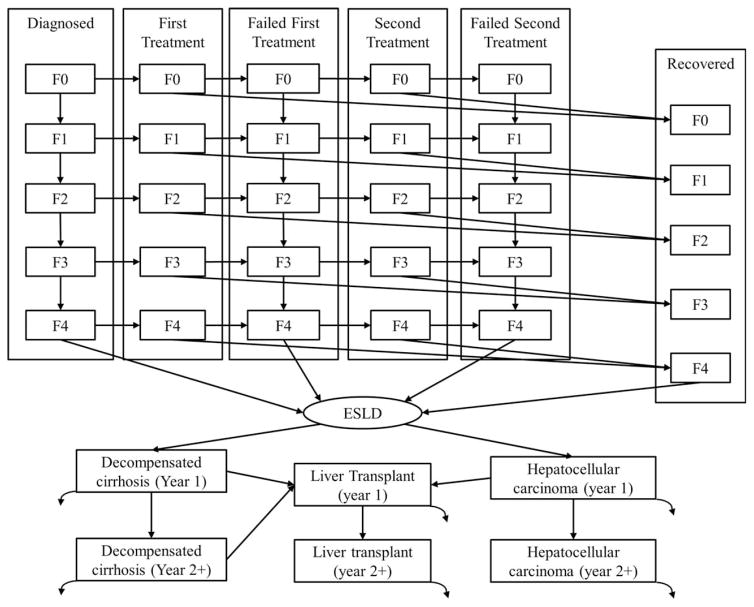
Fig. 1.
Population compartments and liver disease stages in the hepatitis C cost-effectiveness model. The curved arrows exiting ESLD states represent disease-induced mortality rates. Not pictured is that all patients are subject to age-adjusted natural mortality rates (see Supporting Information). “Diagnosed” indicates patients who have been diagnosed with HCV but have not initiated therapy; “First Treatment” indicates patients who are in therapy for HCV for the first time; “Failed First Treatment” indicates patients whose first HCV therapy was not successful and have yet to initiate their second therapy; “Second Treatment” indicates patients who are in therapy for HCV for the second time; “Failed Second Treatment” indicates patients whose first and second HCV therapies were not successful; “Recovered” indicates patients who have recovered from HCV following a successful therapy and are now HCV-uninfected; “ESLD” indicates states that represent end-stage liver disease, which include the first (year 1) and subsequent (year 2+) years of decompensated cirrhosis, hepatocellular carcinoma, and liver transplant.
Because all data were obtained from secondary sources without patient-level information, this study was exempt from human subjects review and approval.
Model Details
We used Microsoft Excel to construct the model. We modeled a closed population of adults who had become chronically infected with HCV prior to the start of the analysis. There was no entry into the population over time, and exit was possible due to death from HCV infection or from other (non-HCV) causes. We used annual time steps such that each year a patient could transition from one state to another or remain in his or her current state, according to the transition probabilities in Table 1. The time horizon of the analysis was the lifetime of the modeled population. We used a societal perspective. All future outcomes, including costs and QALYs, were discounted at 3% annually. All costs were adjusted to 2012 US dollars.
Table 1.
Model Inputs for Hepatitis C Cost-Effectiveness Model*
| Variable/Parameter Description | Relevant Fibrosis or ESLD Stage(s) | Values | Source(s) | ||
|---|---|---|---|---|---|
|
| |||||
| Base Case | Low | High | |||
| State transitions | |||||
| Annual probability of a liver disease stage transition | F0 to F1 | 0.065 | 0.041 | 0.155 | 16,17 |
| F1 to F2 | 0.081 | 0.044 | 0.111 | ||
| F2 to F3 | 0.128 | 0.092 | 0.201 | ||
| F3 to F4 | 0.214 | 0.068 | 0.187 | ||
| Probability of a successful treatment | F0, F1, F2, F3, F4 | 0.900 | 0.500 | 0.999 | 21–24 |
| Annual probability of developing ESLD for HCV-infected patients | F4 to HCC | 0.019 | 0.014 | 0.083 | 13,15 |
| F4 to DC | 0.046 | 0.016 | 0.085 | ||
| Annual probability of developing ESLD for HCV-uninfected patients | F4 to HCC | 0.005 | 0.004 | 0.083 | 13,18,19 |
| F4 to DC | 0.004 | 0.004 | 0.085 | ||
| Annual probability of liver transplant | HCC, DC to LT | 0.023 | 0.010 | 0.031 | 13 |
| Annual probability of liver-related death while in a given ESLD compartment | HCC (year 1) | 0.707 | 0.430 | 0.770 | 13,45 |
| HCC (years 2+) | 0.162 | 0.110 | 0.230 | ||
| DC (year 1) | 0.281 | 0.120 | 0.750 | ||
| DC (years 2+) | 0.281 | 0.120 | 0.750 | ||
| LT (year 1) | 0.107 | 0.100 | 0.210 | ||
| LT (years 2+) | 0.049 | 0.049 | 0.057 | ||
| Discount rate | 0.03 | 0.00 | 0.05 | 46 | |
| Costs (US$2012) | |||||
| Annual costs for nontreatment medical expenses among HCV-infected patients† | F0, F1, F2, F3 | 500 | 200 | 1,800 | 13 |
| F4 | 1,700 | 400 | 19,000 | ||
| Annual costs for nontreatment medical expenses among HCV-uninfected patients | F0, F1, F2, F3 | 400 | 0 | 800 | |
| F4 | 1,700 | 400 | 19,000 | ||
| Annual costs for nontreatment medical expenses among patients with ESLD | HCC (year 1) | 27,400 | 5,100 | 46,900 | 13,45 |
| HCC (year 2+) | 25,200 | 5,100 | 46,900 | ||
| DC (year 1) | 10,900 | 3,000 | 31,200 | ||
| DC (year 2+) | 12,000 | 3,000 | 31,200 | ||
| LT (year 1) | 148,000 | 10,500 | 380,000 | ||
| LT (year 2+) | 10,000 | 2,700 | 27,000 | ||
| Treatment costs | F0, F1, F2, F3, F4 | 100,000 | 50,000 | 150,000 | |
| QALYs | |||||
| Annual QALYs among HCV-uninfected patients | F0, F1, F2, F3 | 0.88 | 0.72 | 1.00 | 13,36 |
| F4 | 0.73 | 0.55 | 0.89 | ||
| Multiplier for HCV-infected patients | F0, F1, F2, F3 | 0.98 | 0.72 | 1.00 | |
| F4 | 0.98 | 0.62 | 1.00 | ||
| Multiplier for patients with ESLD | HCC | 0.52 | 0.10 | 0.91 | |
| DC | 0.82 | 0.51 | 0.91 | ||
| LT | 0.90 | 0.51 | 0.97 | ||
All costs are adjusted to 2012 US dollars and rounded to the nearest hundred. All patients are subject to age-adjusted natural mortality rates (see Supporting Information). Relationships between population compartments and disease stages are depicted in Fig. 1. For additional details of the model and each parameter value see the Supporting Information.
Annual costs of nontreatment medical expenses represent ongoing medical expenses not explicitly considered HCV treatment, such as reoccurring appointments to assess and monitor patient health including a patient’s fibrosis status.
Treatment Status
The model contains population compartments to account for treatment status. Initially, patients are assumed to be treatment-naive. Patients may be treated a second time if their first treatment is unsuccessful. Population compartments include the following: diagnosed, first treatment, failed first treatment, second treatment, failed second treatment, recovered, and three compartments to represent end-stage liver disease (ESLD) (Fig. 1). The ESLD compartments are decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and liver transplant (LT). Recovered patients are HCV-uninfected because they achieved a sustained virologic response following treatment.
Natural History
The staging of liver disease follows the Metavir scale, where F0 represents mild liver disease (or no liver fibrosis) and F4 represents severe liver disease (compensated cirrhosis). Patients in the diagnosed and the failed treatment compartments are subject to the progression of liver disease. Following successful treatment, patients do not progress to higher noncirrhotic liver disease stages. Patients who are cirrhotic (F4) are at risk of developing ESLD (either DC or HCC), but HCV-uninfected patients are at a lower risk than HCV-infected patients (Table 1).
Cost-Effectiveness
Cost-effectiveness ratios compared the costs and health outcomes of two treatment scenarios. The treatment strategies we examined included treatment at F2, at F1, and at F0. The “treatment at F2” strategy initiates treatment if patients have a fibrosis level of F2 or higher. The treatment strategies are increasingly expansive, such that the “treatment at F1” strategy initiates treatment if patients have a fibrosis level of F1 or higher and the “treatment at F0” strategy initiates treatment if patients have a fibrosis level of F0 or higher. We calculated the cost-effectiveness associated with the health care decision to either initiate treatment immediately or delay treatment until a subsequent fibrosis stage (i.e., the cost-effectiveness ratio compares a given treatment strategy with the next least expansive strategy).
Inputs
Liver Disease Progression
Disease progression occurs among precirrhotic liver disease stages (F0–F3) as well as from cirrhosis (F4) to ESLD sequelae (i.e., DC and HCC). Precirrhotic progression rates are applied only to HCV-infected patients who are not in treatment, such that no precirrhotic progression of liver disease occurs among recovered (HCV-uninfected) patients or for the year a patient is in treatment. We chose to use progression rates estimated from CHeCS data as the base case progression rates because the CHeCS provides a large sample of patients that captures the aging US HCV-infected population of greatest interest to this study. Specifically, we applied a stage-specific estimation procedure16 to biopsy records from CHeCS patients who were not coinfected with hepatitis B virus or human immunodeficiency virus and had no other known major comorbidities. This stage-specific procedure yielded separate rates for the transition from F0 to F1, F1 to F2, and so on. The CHeCS-based rates were consistent with other published fibrosis progression rates, well within the upper and lower ranges of those studies16,17 (Table 1). The fibrosis progression rates used in sensitivity analyses were obtained from previously published studies.1,17 For an HCV-infected patient with cirrhosis, the annual probabilities of developing HCC and DC in the base case scenario were assumed to be 1.9% and 4.6%, respectively. These values were based on sources in the literature13,15 and conformed with preliminary analysis of CHeCS data. Relative to HCV-infected patients, HCV-uninfected patients have a 76.4% lower probability of developing HCC18 and 91.3% lower probability of developing DC.19 As with fibrosis progression rates, the range of ESLD-related transition probabilities used in sensitivity analyses were obtained from previously published studies.13
Treatment Effectiveness and Costs
Treatment regimens of direct-acting antivirals that do not burden patients with the adverse events associated with the injection of pegylated interferon have been approved by the US Food and Drug Administration, with more regimens likely to be approved in the future.20 Preliminary evidence suggests success rates greater than 90% for these treatments, even for the more difficult-to-treat genotype 1 patients.21–24 We assumed a base case probability of treatment success of 90% (Table 1).
Since these treatments are relatively new, patient-based, real-world costs are not precisely known. With even more new treatments likely to enter the market in the coming years, the future cost of treatment for HCV infection is difficult to predict. Therefore, we chose to assess the cost-effectiveness of a generic “treatment” at a baseline cost of $100,000 per patient per course, which could conceivably represent any number of emerging pharmaceuticals (or combinations thereof) in addition to any other treatment-associated costs, such as laboratory expenses and outpatient visits. To accommodate the state of substantial uncertainty regarding the current and future costs of HCV treatments, the base case assumption of $100,000 treatment cost was varied extensively in multiple sensitivity analyses.
Chronic Liver Disease Costs and QALYs
As liver fibrosis progresses, health care costs increase because of greater frequency of monitoring and screening as well as greater frequency and costs associated with hospital visits.25 Patients in advanced stages of liver fibrosis also experience a reduction in their quality of life. Accordingly, parameter values for both health care costs and quality of life change dramatically as the patient enters cirrhosis (F4) (Table 1).
Infection with HCV, irrespective of fibrosis level, may impose physical and psychological effects on a patient. For this reason, HCV-infected patients are subjected to a QALY multiplier of 0.98 (Table 1) in the base case, which reduces quality of life of HCV-infected patients by 2% relative to HCV-uninfected persons. This assumption is consistent with previously published cost-effectiveness studies13,26,27 as well as previous studies that measure health-related quality of life.28–30 Even though some studies have found negligible quality-of-life reductions associated with HCV-infected patients when the HCV infection status is unknown to them,31 our model only considers patients who have been diagnosed. Studies have documented the harmful effects of HCV infection on a patient’s psychological well-being32 as well as reductions in health-related quality-of-life measurements following a positive diagnosis.33 Furthermore, a portion of the reduction in quality of life from HCV infection has been documented to rebound, or recover, following successful treatment and a sustained virologic response.34
ESLD Costs and QALYs
Relative to cirrhosis, patients in ESLD incur even greater medical costs and experience lower quality of life. Patients in either DC or HCC may receive LT. All three ESLD states (DC, HCC, and LT) are subdivided into first-year and subsequent-year compartments to allow for different medical costs and disease-related death rates across the years of ESLD (Table 1).
Sensitivity Analyses
Base case model results focus on a 55-year-old HCV-infected patient with a treatment cost of $100,000. In our sensitivity analyses, we vary a wide range of assumptions. Specifically, we performed one-way and multiway sensitivity analyses in which one or more parameter values (or sets of parameter values, such as liver disease stage transitions) were varied at a time, holding all other parameters at their base case values. We conducted a threshold analysis on the treatment cost parameter to estimate the specific treatment cost that yielded cost-effectiveness ratios of $50,000/QALY and $100,000/QALY for patients with no fibrosis (F0). We also computed treatment cost thresholds for several policy-relevant cost-effectiveness levels, stratified by patients who are diagnosed at different fibrosis levels.
Results
Base Case Results
The base case model scenario found that the treatment of patients diagnosed at F2 was generally cost-effective, exhibiting an incremental cost-effectiveness ratio (ICER) of $37,300/QALY (compared to diagnosis at F2 and treatment at F3) (Table 2). At earlier stages of liver disease, the ICER increased to $174,100/QALY and $242,900/QALY, respectively, for patients diagnosed and treated at F1 (compared to being diagnosed at F1 and treated F2) and diagnosed and treated at F0 (compared to being diagnosed at F0 and treated at F2) (Table 2). The treatment of patients who are diagnosed and treated at F0 was compared to that of patients who are diagnosed at F0 and treated at F2 because, for patients diagnosed at F0, treatment at F1 was weakly dominated by treatment at F0.
Table 2.
Discounted Costs, Health Outcomes, and Incremental Cost-Effectiveness Ratios by Treatment Strategy and by Patient Fibrosis Level*
| Patient starting at F0 | Patient starting at F1 | Patient starting at F2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Tx at F3 | Tx at F2 | Tx at F1 | Tx at F0 | Tx at F3 | Tx at F2 | Tx at F1 | Tx at F3 | Tx at F2 | |
| Nontreatment costs ($) | 10,100 | 9300 | 8700 | 7900 | 11,200 | 9100 | 8000 | 12,800 | 8800 |
| Treatment costs ($) | 23,400 | 36,500 | 62,100 | 105,300 | 48,000 | 68,400 | 105,200 | 78,200 | 104,700 |
| Total costs ($) | 33,600 | 45,800 | 70,800 | 113,100 | 59,200 | 77,400 | 113,200 | 91,000 | 113,600 |
| Liver disease deaths | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.03 | 0.01 |
| QALYs | 15.96 | 16.09 | 16.19 | 16.37 | 15.84 | 16.14 | 16.35 | 15.61 | 16.22 |
|
| |||||||||
| Treatment Policies Compared | Tx at F2 Versus Tx at F3 | † | Tx at F0 Versus Tx at F2 | Tx at F2 Versus Tx at F3 | Tx at F1 Versus Tx at F2 | Tx at F2 Versus Tx at F3 | |||
|
| |||||||||
| ICER ($/averted death) | 2,995,000 | 61,326,500 | 1,864,700 | 16,957,300 | 1,211,300 | ||||
| ICER ($/QALY) | 97,900 | 242,900 | 59,500 | 174,100 | 37,300 | ||||
These results were generated using base case parameter values. All costs are presented as 2012 US dollars using a 3% annual discount rate on future costs and benefits. To simplify presentation costs and ICERs, numbers are rounded to the nearest hundred. The results focus on immediate versus delayed treatment (i.e., “treat now” versus “treat later”) for a patient starting at a given level of fibrosis. If “treat later” is not an option, the cost per QALY gained of immediate treatment (versus “never treat”) is $108,800 at F0, $47,000 at F1, and $21,400 at F2 (see Table F1 in the Supporting Information).
This treatment scenario was weakly dominated by the subsequent, more expansive treatment scenario (i.e., in these cases, treatment at F0 dominates treatment at F1 on a cost per QALY basis), and the ICER for treatment at F0 compares treatment at F0 to treatment at F2.
Abbreviation: Tx, treatment.
Sensitivity Analysis
Table 3 reports the ICERs while varying a variety of parameter groups, using the low and high parameter values presented in Table 1. In one-way sensitivity analyses for patients with F2 fibrosis, ICERs range from being cost-saving (<$0/QALY) when nontreatment medical costs are assumed to be high to being as much as $112,300/QALY when liver disease stage transitions are assumed to be low. As expected, the ICERs varied more substantially in the multiway sensitivity analyses. For example, if an HCV patient has liver fibrosis of F1, then treating that patient immediately (treated at F1 compared to treated at F2) generates an ICER that ranges between $16,500/QALY and $665,200/QALY when varying only one parameter at a time and between being cost-saving (<$0/QALY) and $1,807,500/QALY when varying all economic parameters simultaneously (Table 3). Additional multiway sensitivity analyses were also conducted (see Supporting Information).
Table 3.
Results of One-Way and Multiway Sensitivity Analyses*
| Parameter Group Varied | Scenario | Incremental Cost-Effectiveness Ratios ($/QALY) | ||
|---|---|---|---|---|
|
| ||||
| Patient With F0, Tx at F0 Versus F1 | Patient With F1, Tx at F1 Versus F2 | Patient With F2, Tx at F2 Versus F3 | ||
| None | Base case | 242,900‡ | 174,100 | 37,300 |
| Epidemiological parameters | ||||
| Liver disease stage transitions | Low | 288,100‡ | 251,100 | 112,300 |
| High† | 196,100 | 133,700 | 26,400 | |
| Treatment effectiveness | Low | 438,400 | 292,300 | 62,700 |
| High† | 219,900‡ | 160,800 | 34,700 | |
| ESLD transitions | Low | 246,300‡ | 181,700 | 42,900 |
| High† | 234,400 | 127,900 | 16,600 | |
| Disease-induced deaths | Low | 243,900‡ | 176,100 | 37,500 |
| High† | 242,100‡ | 172,800 | 37,100 | |
| Health economic parameters | ||||
| Nontreatment medical costs | Low | 240,300‡ | 172,700 | 39,500 |
| High† | 194,500 | 122,800 | Cost saving | |
| ESLD medical costs | Low | 243,000‡ | 174,600 | 38,900 |
| High† | 242,500‡ | 173,300 | 34,900 | |
| Treatment costs | Low† | 118,900‡ | 84,300 | 15,400 |
| High | 366,800‡ | 263,900 | 59,300 | |
| Quality-of-life assumptions§ | Favor Tx | 19,000‡ | 16,500 | 11,000 |
| Disfavor Tx | 10,860,800 | 665,200 | 46,300 | |
| Discount rate | Low† | 111,500‡ | 54,100 | 3900 |
| High | 343,800 | 266,200 | 70,100 | |
| All epidemiologic parameters | Favor Tx | 158,700 | 79,600 | 11,000 |
| Disfavor Tx | 527,400‡ | 457,900 | 207,300 | |
| All economic parameters | Favor Tx | 300‡ | Cost saving | Cost saving |
| Disfavor Tx | 28,967,600 | 1,807,500 | 144,200 | |
| All parameters | Favor Tx | Cost saving | Cost saving | Cost saving |
| Disfavor Tx | 522,789,800 | 22,539,000 | 1,042,200 | |
This table presents the incremental cost-effectiveness ratios comparing two scenarios under a variety of parameter assumptions. For example, the first value in the row labeled “Liver disease stage transitions/Low” is $288,100, which states that the incremental cost per QALY attained (the incremental cost-effectiveness ratio) for a patient with a starting fibrosis level of F0 is $288,100 when comparing initiating treatment at F0 versus initiating treatment at F1 (i.e., Tx at F0 versus F1). Sensitivity analyses are organized by parameter group, assuming a 55-year-old hepatitis C patient, with treatment of hepatitis C characterized by a generalized all-oral, direct-acting antiviral. When a parameter group was varied, all values in that parameter group were varied simultaneously. For example, in the “Low” scenario for “Liver disease stage transitions,” all values for the “Annual probability of a liver disease stage transition” in Table 1 were set to their low values. Similarly, in the “High” scenario for “treatment effectiveness,” all values for the “Probability of a successful treatment” in Table 1 were set to their high values. The “ESLD transitions” parameter group refers to all values for the “Annual probability of developing ESLD for HCV-infected patients” and “Annual probability of developing ESLD for HCV-infected patients” as listed in Table 1. The “Disease-induced deaths” group refers to all values for the “Annual probability of liver-related death while in a given ESLD compartment” in Table 1. Cost and QALY parameters were varied by group as well. All costs are in 2012 US dollars. To simplify presentation, all numbers were rounded to the nearest hundred.
Indicates this scenario is included in the “favorable” scenarios where multiple parameter groups are varied simultaneously (i.e., “All epidemiologic parameters,” “All economic parameters,” and “All parameters”).
In these scenarios, treatment at F1 is dominated by treatment at F0, so the ICERs presented compare treatment at F0 with treatment at F2.
Within the “Quality-of-life assumptions” scenarios, the favorable scenario uses the high values for QALY (Table 1) associated with being HCV-uninfected and the low value for the QALY multiplier (Table 1), thereby maximizing the difference between quality of life among infected and uninfected populations.
Abbreviation: Tx, treatment.
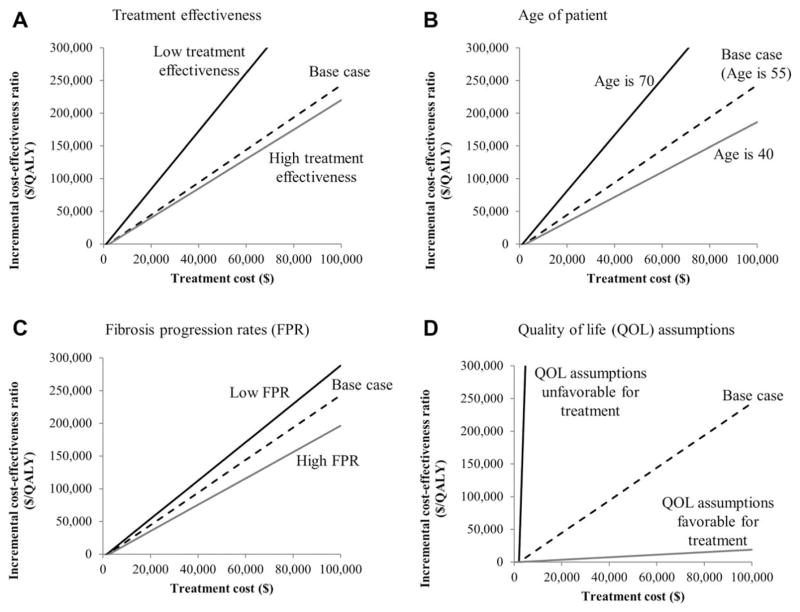
Results from threshold analyses are presented in Fig. 2 and Table 4. For patients with moderate fibrosis (F2), the treatment costs of $14,900, $128,800, and $242,800 yielded, respectively, cost-effectiveness ratios of $0 (or cost saving), $50,000, and $100,000 per QALY gained (Table 4). When we assumed the patient had no evidence of fibrosis (F0), the cost-saving threshold of treatment cost was $2,000 (Table 4). When the cost-effectiveness thresholds were increased to $50,000 and $100,000, the resulting threshold treatment costs for patients with no evidence of fibrosis (F0) increased, respectively, to $22,200 and $42,400 (Table 4).
Fig. 2.
Threshold analyses on treatment cost for HCV-infected patients with no fibrosis where each panel demonstrates the range of parameter values for (A) treatment effectiveness, (B) age of patient, (C) fibrosis progression rates, and (D) quality-of-life assumptions. The lines represent the incremental cost-effectiveness ratios for the treatment of hepatitis C–infected patients at liver disease stage F0 under various assumptions about parameter values. Treatment at F0 is compared to treatment F2 because treatment at F1 is dominated by treatment at F0 for the following scenarios: base case, high treatment effectiveness, age is 70, low fibrosis progression rate, quality-of-life assumptions favorable for treatment. Abbreviations: FPR, fibrosis progression rate; QOL, quality of life.
Table 4.
Hepatitis C Treatment Costs That Yielded Cost-Effectiveness Thresholds Stratified by Fibrosis Level*
| Treatment Scenario | How Is Cost-Effectiveness Defined? | ||
|---|---|---|---|
|
| |||
| $0/QALY† | $50,000/QALY | $100,000/QALY | |
| What treatment cost makes treatment cost-effective for patients regardless of stage?‡ | 2000 | 22,200 | 42,400 |
| What treatment cost makes treatment cost-effective for patients with a fibrosis level of F2 or more severe? | 14,900 | 128,800 | 242,800 |
| What treatment cost makes treatment cost-effective for patients with a fibrosis level of F3 or more severe? | 84,200 | 713,600 | 1,343,000 |
All costs are presented as 2012 US dollars using a 3% annual discount rate on future costs and benefits. Treatment effectiveness was assumed to be 90%, and patients were assumed to be 55 years old.
A cost-effectiveness ratio equal to $0/QALY is commonly used to characterize “cost-saving” interventions.
In this scenario, we computed the threshold treatment cost from the scenario characterized by immediate treatment of a patient at F0 versus delayed treatment at F2 (treatment at F0 dominates treatment at F1).
Discussion
This analysis investigates a common clinical situation, in which the clinician and patient must choose between starting treatment of HCV infection immediately or delaying treatment until later. In the base case scenarios—a 55-year-old patient, treatment cost of $100,000, and treatment effectiveness of 90%—immediate (versus delayed) treatment of a patient with fibrosis level of F0, F1, and F2 was associated with cost-effectiveness ratios, respectively, of $242,900, $174,100, and $37,300 per QALY gained. Earlier initiation of treatment was more cost-effective under scenarios of higher disease progression rates, when quality-of-life assumptions favored treatment, when treatments were more effective, and when lower treatment costs were assumed. We also found that for patients diagnosed and treated at F0, the treatment cost thresholds that yielded $50,000/QALY and $100,000/QALY cost-effectiveness ratios were $22,200 and $42,400, respectively.
Sensitivity analyses showed that results responded to changes in the baseline assumptions about parameter values. In particular, varying the assumption on the impact of a successful treatment on the quality of life for an HCV-infected patient produced relatively large changes in cost-effectiveness and the treatment cost thresholds. This finding highlights the amount of variation that exists given different assumptions about the effect of HCV on a patient’s quality of life in early and late stages of liver disease and underscores a need to better understand the morbidity burden of HCV in both early and late stages of liver disease.
Although we know of no other study that has assessed the cost-effectiveness of HCV treatment considering the stage-specific treatment decision for all the early stages of liver disease, at least two recent cost-effectiveness studies have also investigated the issue of timing of HCV treatment. Younossi et al. 35 found that treating all patients with an all-oral treatment regimen yielded an ICER of $15,700 when compared to treating only patients with F2–F4 fibrosis with an all-oral regimen. Their base case assumptions were more favorable toward a “treat all” strategy than those used in this study. Of particular relevance was that they assumed the difference between the quality of life for an HCV-infected and an HCV-uninfected patient was 10% (or their QALY multiplier for HCV infection was 0.90). Our base case quality-of-life assumptions were more conservative. We reduced quality of life for HCV-infected patients by 2% (or used a QALY multiplier of 0.98), the rationale for which we discuss more extensively in the Supporting Information. Deuffic-Burban et al.36 investigated the decision to treat a patient immediately with interferon-based regimens or to delay treatment until all-oral regimens become available, and they found that delaying treatment was cost-effective for all patients except for those who had already developed cirrhosis. While their analyses differed in that they assumed all-oral regimens were not immediately available, we believe their conclusions were in accordance with ours: delaying treatment for patients who have little evidence of liver damage can be cost-effective if treatment costs are sufficiently high or if disease progression is sufficiently slow, among other factors.
Our results are also generally consistent with other recent assessments of the cost-effectiveness of HCV treatments.3,14,37,38 For example, the incremental cost-effectiveness of triple therapy using direct-acting antivirals (compared to dual therapy) was between $29,200 and $88,900 per QALY.37 Another recent study found the cost-effectiveness of triple therapy (compared to dual therapy) to be between $62,900 and $102,600 per QALY among mildly fibrotic patients and between $32,800 and $54,100 per QALY among patients with advanced fibrosis.38 In similar fashion (without regard to the cost-effectiveness at each fibrosis stage), Hagan et al.14 found that all-oral therapy cost-effectiveness was $44,500/QALY (compared to conventional therapy, which was dual therapy for genotypes 2 and 3 and triple therapy for genotype 1). These studies14,37,38 evaluated different therapy types (comparing all-oral therapy to triple therapy or comparing triple therapy to dual therapy) while assuming a given set of fibrosis levels, whereas our study focused on liver disease stage–specific treatment.
Strategies for HCV testing and linkage to care have been found to be cost-effective in reducing HCV morbidity and mortality.39–42 New therapeutic agents can increase the health benefits associated with these strategies. However, payers and other stakeholders are concerned about their cost and are therefore evaluating these expenditures against the health benefits achieved with these agents. The potential expenditures for HCV screening and treatment strategies are not trivial, given that the United States has approximately 3 million43 HCV-infected persons. Results from our model indicate that HCV therapy appears to be cost-effective for HCV-infected persons with evidence of moderate liver disease.
The findings should be interpreted in light of the limitations of our study. We assumed that HCV-related deaths only occurred as a consequence of developing ESLD, while substantial non–liver-related mortality may be associated with HCV.44 Furthermore, our model does not account for comorbid conditions such as vasculitis and diabetes mellitus, which can be made less severe and life-threatening following successful treatment of HCV infection. The inclusion of these conditions in the model would increase the benefit received from treatment and thereby make the incremental cost-effectiveness ratios associated with treatment more attractive and raise the corresponding treatment cost thresholds. While some studies focusing on injection drug users have found evidence that treatment is a cost-effective intervention to prevent future transmissions,45 we assume no such benefits occurred after successful treatment. Although alcohol consumption was not explicitly accounted for in our modeling of liver fibrosis progression, using the fibrosis progression rates estimated from the CHeCS, due to the CHeCS cohort size and heterogeneity in terms of racial, geographic, and economic characteristics, a mixture of alcohol consumption behaviors are likely represented. Considering these limitations, we provided ranges on all parameters and designed broad sensitivity analyses to capture scenarios that both favored and disfavored treatment cost-effectiveness. A literal interpretation of the model supposes that fibrosis status among HCV-infected patients is known with a high level of sensitivity and specificity, when in fact neither noninvasive methods nor biopsies can ascertain a liver’s fibrotic status with perfect accuracy; additionally, subjecting a patient to repeated liver biopsies to evaluate liver histology could evoke ethical concerns. Similarly, we also assumed that delayed treatment is a viable option for all patients at F0, F1, and F2. The cost-effectiveness of immediate treatment is more favorable if delayed treatment is not an option (see Supporting Information).
In summary, treatment of HCV patients diagnosed with moderate to severe liver disease (F2–F4) was found to be cost-effective. Earlier treatment can be a cost-effective use of resources in some scenarios and with certain thresholds of treatment costs. In the current era of evolving antiviral therapy for HCV infection, these results can help to inform policies that guide initiation of therapy.
Supplementary Material
Acknowledgments
We are grateful for the research assistance of Anne Moorman, Xin Tong, Jing Xing, and the investigators of the Chronic Hepatitis Cohort Study. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The Chronic Hepatitis Cohort Study is funded by the Centers for Disease Control Foundation, which received grants from AbbVie; Abbott Laboratories; Genetech, a Member of the Roche Group; Janssen Pharmaceutical Companies of Johnson & Johnson; and Vertex Pharmaceuticals. Granting corporations do not have access to study data and do not contribute to data analysis or the writing of manuscripts.
Abbreviations
- CHeCS
Chronic Hepatitis Cohort Study
- DC
decompensated cirrhosis
- ESLD
end-stage liver disease
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ICER
incremental cost-effectiveness ratio
- LT
liver transplant
- QALY
quality-adjusted life-year
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.27736/suppinfo.
References
- 1.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in US primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2000;133:665–675. doi: 10.7326/0003-4819-133-9-200011070-00008. [DOI] [PubMed] [Google Scholar]
- 4.Xu F, Tong X, Leidner AJ. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health Affairs. 2014;33:1728–1735. doi: 10.1377/hlthaff.2014.0096. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk ML, Tocco R, Saini S, Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 7.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Hepatitis B and C virus infection among 1. 2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–1055. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt AA, McGinnis KA, Skanderson M, Justice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int. 2010;30:240–250. doi: 10.1111/j.1478-3231.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Association for the Study of Liver Diseases/Infectious Diseases Society of America/International Antiviral Society-USA. [Accessed August 15, 2014];Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org.
- 10.Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, Lu M, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis. 2013;56:40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijayadeva V, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus: patients in a large US cohort. Clin Infect Dis. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbasha EH, Chhatwal J, Ferrante SA, El Khoury AC, Laires PA. Cost-effectiveness analysis of boceprevir for the treatment of chronic hepatitis C virus genotype 1 infection in Portugal. Appl Health Econ Health Policy. 2013;11:65–78. doi: 10.1007/s40258-012-0007-8. [DOI] [PubMed] [Google Scholar]
- 13.Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14:1068–1077. doi: 10.1016/j.jval.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepatitis. 2013;20:847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis CA cost-effectiveness analysis. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi Q, Wang P, Krahn M. Improving the accuracy of long-term prognostic estimates in hepatitis C virus infection. J Viral Hepatitis. 2004;11:166–174. doi: 10.1046/j.1365-2893.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 17.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 18.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufor JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis: sustained virological response and all-cause mortality. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 20.Pollack A. New York Times. Dec 6, 2013. F.D.A. approves pill to treat hepatitis C. [Google Scholar]
- 21.Lawitz E, Poordad F, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 22.Lawitz E, Vierling JM, Murillo A, Kugelmas M, Gerstoff J, Balart LA, et al. High efficacy and saftety of the all-oral combination regimen, Mk-5172/MK-8742 +/− RBV for 12 weeks in HCV genotype 1 infected patients: the C-Worthy study. Presented at: The Liver Meeting: The 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 3, 2013; Washington DC. [Google Scholar]
- 23.Lawitz E, Hezode C, Varunok P, Thuluvath J, Baykal T, Kapoor M, et al. Interferon-and ribavirin-free regimen of ABT-450/r +ABT-267 in HCV genotype 1b–infected treatment-naive patients and prior null responders. Presented at: The Liver Meeting: The 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 3, 2013; Washington DC. [Google Scholar]
- 24.Jacobson IM, Ghalib RH, Rodriguez-Torres M, Younossi ZM, Corregidor A, Sulkowski MS, et al. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study. Presented at: The Liver Meeting: The 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 3, 2013; Washington DC. [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C. JAMA. 1998;280:2088–2093. doi: 10.1001/jama.280.24.2088. [DOI] [PubMed] [Google Scholar]
- 27.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-α2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Carithers RL, Jr, Sugano D, Bayliss M. Health assessment for chronic HCV infection. Dig Dis Sci. 1996;41:75S–80S. doi: 10.1007/BF02087879. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 30.Foster G, Goldin R, Thomas H. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 31.Schwarzinger M, Dewedar S, Rekacewicz C, Abd Elaziz KM, Fontanet A, Carrat F, et al. Chronic hepatitis C virus infection: does it really impact health-related quality of life? A study in rural Egypt. Hepatology. 2004;40:1434–1441. doi: 10.1002/hep.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castera L, Constant A, Bernard P-H, de Ledinghen V, Couzigou P. Psychological impact of chronic hepatitis C: comparison with other stressful life events and chronic diseases. World J Gastroenterol. 2006;12:1545. doi: 10.3748/wjg.v12.i10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–1301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 35.Younossi ZM, Mendel ES, Heshaam MM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60:530–537. doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Deuffic-Burban S, Schwarzinger M, Obach D, Mallet V, Pol S, Pageaux GP, et al. Should we await INF-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis. J Hepatol. 2014;61:7–14. doi: 10.1016/j.jhep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Chan K, Lai MN, Groessl EJ, Hanchate AD, Wong JB, Clark JA, et al. Cost-effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2013;11:1503–1510. doi: 10.1016/j.cgh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwan P, Ward T, Yuan Y, Kim R, L’Italien G. The impact of timing and prioritization on the cost-effectiveness of birth-cohort testing and treatment for hepatitis C virus in the US. Hepatology. 2013;58:54–64. doi: 10.1002/hep.26304. [DOI] [PubMed] [Google Scholar]
- 40.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–1355. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 41.Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the US. Clin Infect Dis. 2013;56:1382–1393. doi: 10.1093/cid/cit069. [DOI] [PubMed] [Google Scholar]
- 42.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–301. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, et al. Mortality among persons in care with hepatitis C virus infection—the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clin Infect Dis. 2014;58:1055–1061. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 44.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razavi H, El Khoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.