Abstract
Studies of ancient human skeletal remains frequently proceed from the assumption that individuals with robust limb bones and/or rugose, hypertrophic entheses can be inferred to have been highly physically active during life. Here, we experimentally test this assumption by measuring the effects of exercise on limb bone structure and entheseal morphology in turkeys. Growing females were either treated with a treadmill-running regimen for 10 weeks or served as controls. After the experiment, femoral cortical and trabecular bone structure were quantified with μCT in the mid-diaphysis and distal epiphysis, respectively, and entheseal morphology was quantified in the lateral epicondyle. The results indicate that elevated levels of physical activity affect limb bone structure but not entheseal morphology. Specifically, animals subjected to exercise displayed enhanced diaphyseal and trabecular bone architecture relative to controls, but no significant difference was detected between experimental groups in entheseal surface topography. These findings suggest that diaphyseal and trabecular structure are more reliable proxies than entheseal morphology for inferring ancient human physical activity levels from skeletal remains.
Keywords: Muscle attachment site, Muscle force, Cortical bone, Trabecular bone, Exercise
1. Introduction
Physical activity levels are a critical aspect of human biological and cultural variation (Leonard, 2008; Pontzer et al., 2012). Physical activity is essential for individual health and survival (Lieberman, 2013), it can be a driving force behind phenotypic evolution (Wallace et al., 2010; Raichlen and Polk, 2013), and it is a nexus that links features of economy, technology, and social relations (Kelly, 2013). For these reasons, paleoanthropological investigations of the lifeways of our ancient ancestors often aim to glean information about their levels of physical activity, typically by analyzing their skeletal remains (e.g., Villotte et al., 2010; Lieverse et al., 2013; Shaw and Stock, 2013; Chirchir et al., 2015; Ruff et al., 2015). Two skeletal features are assumed to be especially informative: (1) the quantity and distribution of bone within the limb elements, and (2) the morphology of muscle and tendon attachments sites, or entheses (Jurmain et al., 2012; Larsen, 2015). Individuals with robust limb bones and/or rugose, hypertrophic entheses are inferred to have been highly active during life, whereas individuals with gracile limb bones and/or smooth, modest-sized entheses are assumed to have been more sedentary.
In this study, we experimentally test the model within which paleoanthropologists deduce physical activity levels from human skeletal remains by measuring the effects of treadmillrunning exercise on limb bone structure and entheseal morphology in turkeys. If the paleoanthropological model is valid, then animals treated with exercise should exhibit enhanced bone structure and/or altered entheseal form. Turkeys and other bipedal birds are good locomotor models for human locomotion because the species exhibit similar patterns of limb movement (Gatesy and Biewener, 1991), basic body center-of-mass dynamics and pendular exchange (Biewener and Daley, 2007), and energetic costs associated with locomotion (Rubenson et al., 2006). Bipedal birds are also a well-accepted system for investigating links between activity-induced bone loading, limb muscle stresses, and bone functional adaptation (e.g., Roberts et al., 1997; Judex and Zernicke, 2000; Gabaldon et al., 2004; Pontzer et al., 2006).
2. Methods
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Brown University. The experiment was designed and carried out for an unrelated study. Thus, no animals were harmed for the specific purpose of gathering the data reported here.
Ten 1-year-old female Eastern wild turkeys (Meleagris gallopavo) were obtained from a licensed breeder and transported to the animal care facility at Brown University. Animals were group-housed in a well-ventilated, climate-controlled room on a 12:12 light:dark cycle. The room was fitted with a pen (6 × 10 × 3 m; width × length × height) with rubber flooring. Hay was provided for bedding, and food and water were available ad libitum. When left undisturbed in the pen, all animals were generally quiescent and active only for brief periods for socialization, eating, and drinking, similar to the behavior of turkeys observed in prior studies (Adams et al., 1997; Fritton et al., 2000).
Animal subjects were designated as either runners or non-runner controls (n = 5/group). Runners were exercised on a declined motor-driven treadmill at a speed of 2.5 m s−1 for 30 min a day, four days a week, for 10 weeks, on average taking 141,912 (standard deviation = 7,280) strides during the exercise treatment. Each stride generated peak vertical substrate reaction forces between approximately 150% and 250% of the animal’s body weight (Roberts and Scales, 2002). This exercise routine constitutes a substantial increase in high-magnitude limb loading events relative to the normally sedentary activity patterns of laboratory turkeys (Fritton et al., 2000), and it is consistent with prior exercise studies that have revealed statistically significant musculoskeletal tissue changes in galliform birds (Buchanan and Marsh, 2001, 2002), as well as in mammals (Wallace et al., 2015). All subjects were euthanized by 18–19 months of age and their femora were extracted. The use of animals between one and two years old is ideal for examining the skeletal effects of physical activity, as the adaptability of turkey bones to mechanical loading has been shown to be high during this ontogenetic period (Rubin et al., 1992).
Femora were scanned at a 45-μm3 voxel size using a Nikon XT H 225 ST μCT system. Cortical and trabecular bone structural properties were quantified from μCT images using the BoneJ plugin (Doube et al., 2010) for ImageJ software. Images were thresholded to extract the bone phase using the “optimise threshold” option in BoneJ. Cortical bone parameters were measured from mid-diaphyseal transverse cross-sectional μCT images and included cortical bone area, maximum second moments of area, and minimum second moments of area. In standard beam analysis, cortical area approximates a bone cross section’s internal resistance to axial compression and tension, and maximum and minimum second moments of area describe resistance to bending around principal axes. Trabecular bone structural properties were quantified in the lateral condyles of distal femoral epiphyses. This location was chosen for analysis due to its close proximity to the entheseal site examined (see below). Volumes of interest (VOI) were defined in μCT image stacks as the largest centered sphere to fit completely within each region of trabecular bone. Bone volume fraction is the percentage of bone voxels present in the VOI relative to the total number of voxels in the VOI. Trabecular thickness is the average strut thickness. Trabecular connectivity density characterizes the redundancy of trabecular connections, normalized to the volume of the VOI.
Entheseal morphology was assessed at the lateral epicondyles of distal femora (Fig. 1). The lateral epicondyle is the attachment site for the tendon of the lateral gastrocnemius muscle, a key extensor of the ankle. This site was selected for analysis because during decline running in turkeys, the lateral gastrocnemius is activated at high levels (generating forces >70% of the animal’s body weight) to function as a brake, a mechanical function that is facilitated by muscle fibers undergoing lengthening contractions during stance in order to absorb mechanical energy (Gabaldon et al., 2004; Roberts and Azizi, 2010). Thus, if muscle activity associated with decline running influences turkey entheseal morphology, it is reasonable to expect that the lateral epicondyles would be especially affected.
Figure 1.
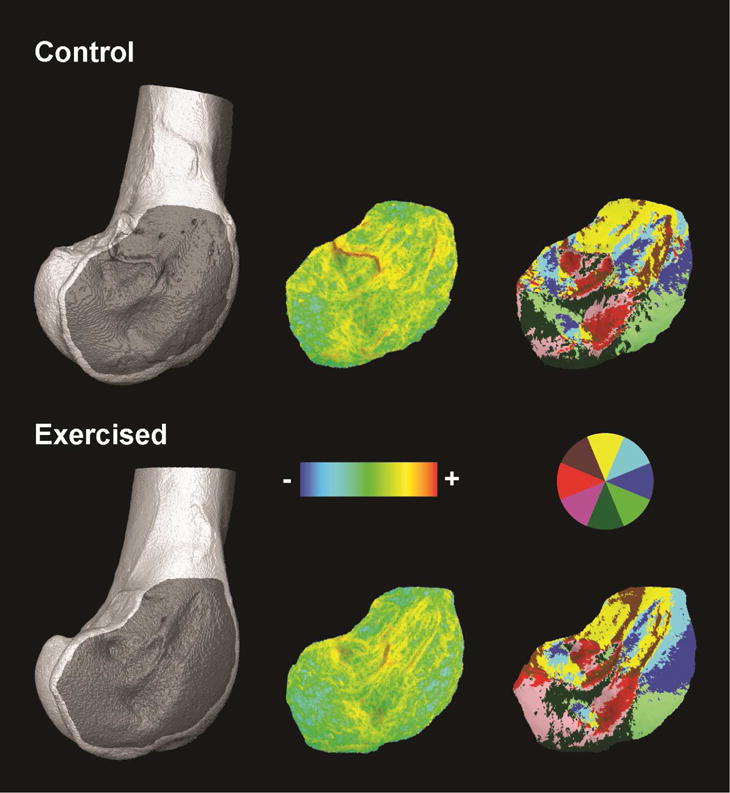
Lateral epicondyles of femora from a control animal and an exercised animal. Left: μCT renderings of distal femora with darker gray regions indicating entheseal surfaces isolated for topographic analyses. Middle: Dirichlet normal energy (curvature) illustrated using a logarithmic color scale standardized across surfaces. Hotter colors indicate greater degrees of local bending across the surface. Right: Orientation patch count rotated (complexity) illustrated by coloring surface polygons by the direction they face in the epicondyle surface plane. A color compass is provided for reference.
The shape of entheseal surfaces was quantified using morphological topographic analysis (Evans, 2013). Topographic metrics analyzed included Dirichlet normal energy (which describes surface curvature [Bunn et al., 2011]), relief index (which describes surface relief [M’Kirera and Ungar, 2003; Boyer, 2008]), and orientation patch count rotated (which describes surface complexity [Evans et al., 2007]) (Fig. 1). Quantifying these aspects of shape together provides a holistic assessment of surface morphology, as has been well illustrated in studies of tooth shape (e.g., Bunn et al., 2011; Winchester et al., 2014).
For topographic analyses, Amira software was used to segment 3D external surfaces of distal femora from μCT images, and then to crop the surfaces to isolate the lateral epicondyles (Fig. 1). In order to ensure comparability of lateral epicondyle surfaces between animals, a standard cropping protocol was developed. Lateral epicondyle morphology can be generally described as laterally convex with limited degrees of gentle curvature leading to sharper flexure around the anterior, posterior, and inferior borders. Anterior, posterior, and inferior margins of epicondyle surfaces were placed at the lines of transition between blunter and sharper degrees of curvature. Junctions between posterior and superior margins were placed along posterior margins at heights equal to the furthest observed extents of the gastrocnemius entheses, and superior margins were placed as straight lines from posterior to anterior margins. All polygons within the defined margins were cropped to produce isolated epicondyle surfaces. Surfaces were then simplified to 20,000 polygon faces and smoothed with a single iteration prismatic shapes (aggressive) method using Geomagic software.
After epicondyle surfaces were cropped, simplified, and smoothed, topographic variables were quantified using the MorphoTester application (Winchester, 2016). Dirichlet normal energy was measured as a sum of per-polygon curvature or bending values across the epicondyle surface. Bending per polygon is quantified as the change in normal vector map relative to change in polygon vertices, multiplied by polygon area to account for polygon scaling (for more detail see Bunn et al., 2011; Winchester, 2016). MorphoTester parameters for Dirichlet normal energy calculation included condition number checking and outlier removal of energy times polygon area values at 99.9th percentile. Implicit fair smoothing was not used. Relief index was calculated as the natural log of the square root of surface area divided by the square root of projection area, resulting in a dimensionless quantity. Orientation patch count rotated was measured as a count of patches across the epicondyle surface with differing facing or aspect on the epicondyle surface plane, averaged across eight rotations to account for rotation of that plane around a central perpendicular axis (approximately mediolateral here). This topographic variable was measured with a minimum patch count of six, meaning that surface patches comprising fewer than six polygons are not counted toward the surface patch total.
Shapiro-Wilk tests were used to determine if data followed a normal distribution, and Levene’s tests were used to assess the equality of group variances. Depending on the results of these tests, differences between the two experimental groups were analyzed with independent samples t-tests, Mann-Whitney U-tests, or Welch’s t-tests. Significance level for tests was p<0.05 and tests were two-tailed.
3. Results
At the end of the experimental period, body mass was not significantly different between groups, nor was femur length, indicating that exercise treatment had little effect on overall body size (Table 1). Significant group differences were, however, found in features of limb bone structure (Table 1). On average, animals that engaged in treadmill running had femoral diaphyses with 44% larger minimum second moments of area (p=0.03), reflecting enhanced resistance to bending in approximately the shaft’s anteroposterior plane. Exercised animals also had, on average, 17% thicker trabecular bone (p=0.02), resulting in a 21% increase in trabecular bone volume fraction in the distal femur (p=0.03). In contrast, no significant group differences were detected in any of the entheseal morphometric parameters analyzed (Table 1; Fig. 1), including surface curvature, surface relief, and surface complexity.
Table 1.
Body size and bone structural properties in controls and exercised animalsa
| Trait | Control | Exercised | p |
|---|---|---|---|
| Body size | |||
| Body mass (kg) | 3.31 (0.14) | 3.46 (0.36) | 0.45 |
| Femur length (mm) | 110.2 (3.8) | 113.0 (2.6) | 0.22 |
| Cortical bone | |||
| Cortical area (mm2) | 28.9 (2.4) | 36.2 (11.6) | 0.22 |
| Maximum second moments of area (mm4) | 335.2 (51.4) | 417.7 (152.3) | 0.42 |
| Minimum second moments of area (mm4) | 262.2 (29.9) | 376.7 (120.1) | 0.0317 |
| Trabecular bone | |||
| Bone volume fraction (%) | 12.5 (1.6) | 15.1 (1.4) | 0.027 |
| Trabecular thickness (mm) | 0.303 (0.024) | 0.353 (0.028) | 0.017 |
| Connectivity density (1/mm3) | 0.646 (0.436) | 0.468 (0.089) | 0.40 |
| Entheseal morphology | |||
| Dirichlet normal energy | 101.1 (6.7) | 125.4 (67.4) | 0.47 |
| Relief index | 0.020 (0.004) | 0.020 (0.006) | 0.98 |
| Orientation patch count rotated (patches) | 186.8 (15.5) | 202.2 (45.4) | 0.51 |
Values are means (standard deviations). Values in bold are statistically significant.
4. Discussion
The results of this experiment indicate that variation in physical activity influences the structure of turkey hind limb bones but not entheseal morphology. Specifically, animals subjected to treadmill-running exercise displayed enhanced femoral diaphyseal and trabecular bone architecture relative to controls, whereas no significant difference was detected between experimental groups in femoral entheseal surface topography. Extrapolating these results from turkeys to humans requires caution due to biological differences between the species (e.g., genetics, body size, metabolism, bone tissue composition), and the influence of these differences on experimental data is currently unknown. Nevertheless, similarities between human and bipedal bird locomotion (e.g., Gatesy and Biewener, 1991; Rubenson et al., 2006; Biewener and Daley, 2007) and the species’ skeletal response to mechanical signals (Rubin and Lanyon, 1984; Pontzer et al., 2006) confer on the turkey model a compelling degree of face validity. If extrapolation is indeed warranted, then the data presented here suggest that diaphyseal and trabecular structure are more reliable proxies than entheseal morphology for inferring ancient human activity levels from skeletal remains.
Our findings accord with those of many previous experiments involving animal models that examined the effects of elevated physical activity on limb bone diaphyseal and trabecular structure (reviewed in Wallace et al., 2017). In a diversity of animals including rodents (e.g., Wallace et al., 2015), ruminants (e.g., Lieberman et al., 2003; Barak et al., 2011), swine (e.g., Lieberman, 1996), and now turkeys, loadbearing forms of exercise such as running, particularly if they occur during the growing years, have been shown to enhance bone formation and augment diaphyseal robusticity and trabecular architecture, providing empirical support for the model within which paleoanthropologists commonly infer ancient human activity levels from these structural features of limb bone remains. Diaphyseal and trabecular structure are strongly affected by intrinsic factors such as age, sex, and genetics, but in studies of human skeletal remains in which these non-mechanical factors can be carefully evaluated and convincingly argued to not underlie observed morphological patterns, ample evidence from animal experiments suggests that such patterns may be due to variation in physical activity levels.
Our results are also consistent with those of the only two previous experiments that have assessed the effects of exercise on entheseal morphology. In the first study, Zumwalt (2006) exercised sheep on a treadmill for 60 min/day for 90 days while they carried additional weight (20% of body mass) on their backs. At the end of the treatment period, the size and complexity of entheses at multiple limb bone sites were quantified and found to not be significantly different from those in a group of unexercised controls. This lack of an exercise effect was attributed to the old age of the animals employed, in which entheses were perhaps less adaptable to activity-induced loads. Similarly, Rabey and colleagues (2015) showed that 11 weeks of voluntary wheel running and tower climbing in mice had little effect on humerus deltoid crest size relative to normal cage activity. As in our study, the mice were still growing during the start of the experiment, making ontogeny an unlikely explanation for the absence of an activity effect on entheseal morphology. To our knowledge, no experimental or longitudinal study has ever documented a significant change in entheseal morphology resulting from engagement in an exercise regimen. Until direct evidence exists for a link between physical activity and entheseal morphology, we suggest that entheses should not be used to infer ancient human activity levels.
Reconstructing the physical activity levels of our ancient human ancestors represents a major goal for paleoanthropology. Because human skeletal remains are among the richest sources of data for paleoanthropologists, the value of skeletal indicators of physical activity is readily apparent. Nevertheless, this study underscores the importance of experimentally testing the causal relationships between physical activity and bony anatomy assumed in studies of human skeletal remains. By suggesting that entheses should not be considered a reliable proxy for physical activity, we are not implying that researchers restrict their scope of analysis to only diaphyseal and trabecular structure. On the contrary, we believe that inferences about the activity levels of our ancient ancestors should be based on as many lines of evidence as possible, including abundant data provided by the archeological record. However, it is important to recognize that some measures of physical activity are more substantiated than others and thus warrant greater consideration and research effort. Experiments can greatly contribute to the epistemology of paleoanthropological inquiry by helping to identify which measures allow us to most faithfully reconstruct ancient human activity from the fossil and archeological records.
Acknowledgments
This work was supported by NIH AR055295 (to Dr. Thomas Roberts, Brown University) and NSF BCS1341120 (to J.M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J Biomechanics. 1997;30:671–678. doi: 10.1016/s0021-9290(97)00004-3. [DOI] [PubMed] [Google Scholar]
- Barak MM, Lieberman DE, Hublin JJ. A Wolff in sheep’s clothing: trabecular bone adaptation in response to changes in joint loading orientation. Bone. 2011;49:1141–1151. doi: 10.1016/j.bone.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Daley MA. Unsteady locomotion: integrating muscle function with whole body dynamics and neuromuscular control. J Exp Biol. 2007;210:2949–2960. doi: 10.1242/jeb.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer DM. Relief index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. J Hum Evol. 2008;55:1118–1137. doi: 10.1016/j.jhevol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90:164–171. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1101–1107. doi: 10.1016/s1095-6433(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Bunn JM, Boyer DM, Lipman Y, St Clair EM, Jernvall J, Daubechies I. Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. Am J Phys Anthropol. 2011;145:247–261. doi: 10.1002/ajpa.21489. [DOI] [PubMed] [Google Scholar]
- Chirchir H, Kivell TL, Ruff CB, Hublin JJ, Carlson KJ, Zipfel B, Richmond BG. Recent origin of low trabecular bone density in modern humans. Proc Natl Acad Sci. 2015;112:366–371. doi: 10.1073/pnas.1411696112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doube M, Klosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR. Shape descriptors as ecometrics in dental ecology. Hystrix. 2013;24:133–140. [Google Scholar]
- Evans AR, Wilson GP, Fortelius M, Jernvall J. High-level similarity of dentitions in carnivorans and rodents. Nature. 2007;445:78–81. doi: 10.1038/nature05433. [DOI] [PubMed] [Google Scholar]
- Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomechanics. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- Gabaldon AM, Nelson FE, Roberts TJ. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J Exp Biol. 2004;207:2277–2288. doi: 10.1242/jeb.01006. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Biewener AA. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J Zool. 1991;224:127–147. [Google Scholar]
- Judex S, Zernicke RF. Does the mechanical milieu associated with high-speed running lead to adaptive changes in diaphyseal growing bone? Bone. 2000;26:153–159. doi: 10.1016/s8756-3282(99)00256-2. [DOI] [PubMed] [Google Scholar]
- Jurmain R, Cardoso FA, Henderson C, Villotte S. Bioarchaeology’s holy grail: the reconstruction of activity. In: Grauer AL, editor. A Companion to Paleopathology. Wiley-Blackwell; West Sussex: 2012. pp. 532–552. [Google Scholar]
- Kelly RL. The Lifeways of Hunter-Gatherers: The Foraging Spectrum. Cambridge University Press; New York: 2013. [Google Scholar]
- Larsen CS. Bioarchaeology: Interpreting Behavior from the Human Skeleton. Cambridge University Press; Cambridge: 2015. [Google Scholar]
- Leonard WR. Lifestyle, diet, and disease: comparative perspectives on the determinants of chronic health risks. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. Oxford University Press; Oxford: 2008. pp. 265–276. [Google Scholar]
- Lieberman DE. How and why humans grow thin skulls: experimental evidence for systemic cortical robusticity. Am J Phys Anthropol. 1996;101:217–236. doi: 10.1002/(SICI)1096-8644(199610)101:2<217::AID-AJPA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lieberman DE. The Story of the Human Body: Evolution, Health, and Disease. Pantheon; New York: 2013. [PubMed] [Google Scholar]
- Lieberman DE, Pearson OM, Polk JD, Demes B, Crompton AW. Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J Exp Biol. 2003;206:3125–3138. doi: 10.1242/jeb.00514. [DOI] [PubMed] [Google Scholar]
- Lieverse AR, Bazaliiskii VI, Goriunova OI, Weber AW. Lower limb activity in the Cis-Baikal: entheseal changes among Middle Holocene Siberian foragers. Am J Phys Anthropol. 2013;150:421–432. doi: 10.1002/ajpa.22217. [DOI] [PubMed] [Google Scholar]
- M’Kirera F, Ungar PS. Occlusal relief changes with molar wear in Pan troglodytes troglodytes and Gorilla gorilla gorilla. Am J Primatol. 2003;60:31–41. doi: 10.1002/ajp.10077. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Lieberman DE, Momin E, Devlin MJ, Polk JD, Hallgrímsson B, Cooper DML. Trabecular bone in the bird knee responds with high sensitivity to changes in load orientation. J Exp Biol. 2006;209:57–65. doi: 10.1242/jeb.01971. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Wood BM, Mabulla AZP, Racette SB, Marlowe FW. Hunter-gatherer energetics and human obesity. PLoS ONE. 2012;7:e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabey KN, Green DJ, Taylor AB, Begun DR, Richmond BG, McFarlin SC. Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. J Hum Evol. 2015;78:91–102. doi: 10.1016/j.jhevol.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Polk JD. Linking brains and brawn: exercise and the evolution of human neurobiology. Proc Roy Soc B. 2013;280:20122250. doi: 10.1098/rspb.2012.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Azizi EA. The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J Appl Physiol. 2010;109:396–404. doi: 10.1152/japplphysiol.01272.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Scales JA. Mechanical power output during running accelerations in wild turkeys. J Exp Biol. 2002;205:1485–1494. doi: 10.1242/jeb.205.10.1485. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Rubenson J, Henry HT, Dimoulas PM, Marsh RL. The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris) J Exp Biol. 2006;209:2395–2408. doi: 10.1242/jeb.02310. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Jt Surg. 1984;66A:397–402. [PubMed] [Google Scholar]
- Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50:306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Holt B, Niskanen M, Sladek V, Berner M, Garofalo E, Garvin HM, Hora M, Junno JA, Schuplerova E, Vilkama R, Whittey E. Gradual decline in mobility with the adoption of food production in Europe. Proc Natl Acad Sci. 2015;112:7147–7152. doi: 10.1073/pnas.1502932112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TM, Ketcham RA. The three-dimensional structure of trabecular bone in the femoral head of strepsirrhine primates. J Hum Evol. 2002;43:1–26. doi: 10.1006/jhev.2002.0552. [DOI] [PubMed] [Google Scholar]
- Shaw CN, Stock JT. Extreme mobility in the Late Pleistocene? Comparing limb biomechanics among fossil Homo, varsity athletes and Holocene foragers. J Hum Evol. 2013;64:242–249. doi: 10.1016/j.jhevol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Villotte S, Churchill SE, Dutour O, Henry-Gambier D. Subsistence activities and the sexual division of labor in the European Upper Paleolithic and Mesolithic: evidence from upper limb enthesopathies. J Hum Evol. 2010;59:35–43. doi: 10.1016/j.jhevol.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Wallace IJ, Middleton KM, Lublinsky S, Kelly SA, Judex S, Garland T, Jr, Demes B. Functional significance of genetic variation underlying limb bone diaphyseal structure. Am J Phys Anthropol. 2010;143:21–31. doi: 10.1002/ajpa.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Pagnotti GM, Rubin-Sigler J, Naeher M, Copes LE, Judex S, Rubin CT, Demes B. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J Exp Biol. 2015;218:3002–3009. doi: 10.1242/jeb.118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Demes B, Judex S. Ontogenetic and genetic influences on bone’s responsiveness to mechanical signals. In: Richtsmeier JT, Percival CJ, editors. Building Bones: Bone Formation and Development in Anthropology. Cambridge University Press; Cambridge: 2017. pp. 233–253. [Google Scholar]
- Winchester JM. MorphoTester: an open source application for morphological topographic analysis. PLOS ONE. 2016;11:e0147649. doi: 10.1371/journal.pone.0147649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester JM, Boyer DM, St Clair EM, Gosselin-Ildari AD, Cooke SB, Ledogar JA. Dental topography of platyrrhines and prosimians: convergence and contrasts. Am J Phys Anthropol. 2014;153:29–44. doi: 10.1002/ajpa.22398. [DOI] [PubMed] [Google Scholar]
- Zumwalt A. The effect of endurance exercise on the morphology of muscle attachment sites. J Exp Biol. 2006;209:444–454. doi: 10.1242/jeb.02028. [DOI] [PubMed] [Google Scholar]


