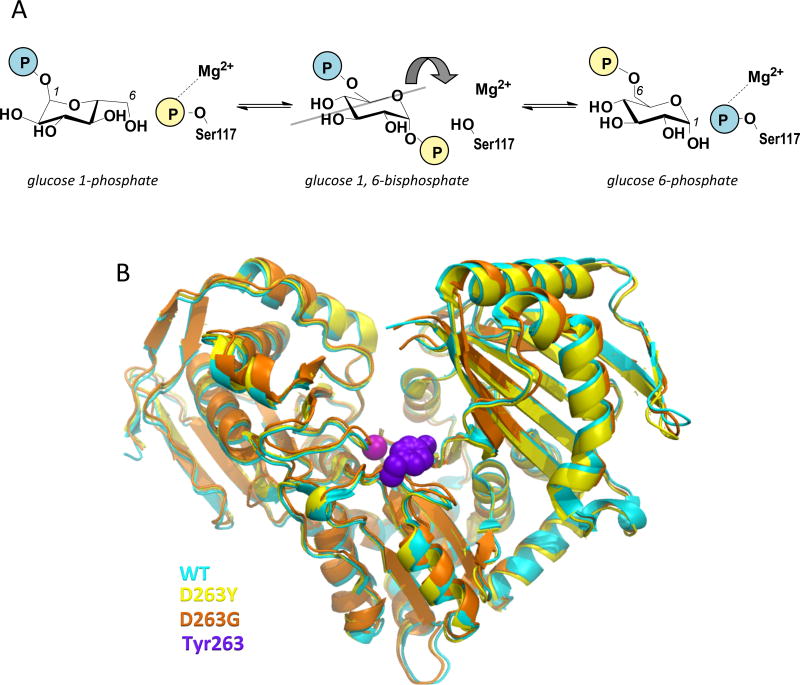
Figure 1. Overview of the mechanism and structure of human PGM1.
(A) A schematic of the catalytic reaction, showing the reversible conversion of glucose 1-phosphate to glucose 6-phosphate. The glucose 1,6-bisphosphate intermediate undergoes a 180° reorientation in between the two phosphoryl transfer steps of the reaction (gray line indicates axis of rotation).
(B) A superposition showing the overall similarity between the structures of WT human PGM1 and the D263G and D263Y missense variants. The WT structure is shown in cyan, D263G in orange, and the D263Y in yellow. The bound metal ion as shown as a magenta sphere and the side chain of Tyr263 with purple spheres.