Fig. 2.
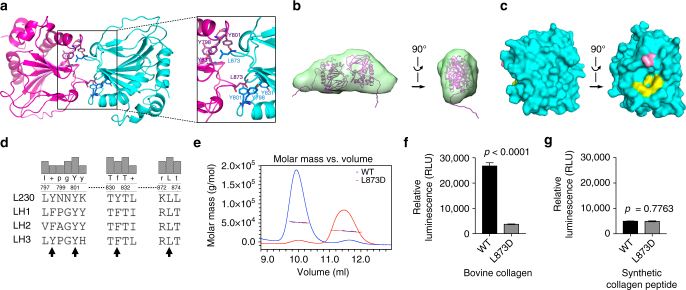
The LH domain forms a homodimer in a tail-to-tail orientation through a unique interface. a Ribbon diagram of the L230 LH domain homodimer. Key residues on the interface are indicated (inset). b A tail-to-tail homodimer detected by small-angle X-ray scattering (SAXS) analysis of L230 LH domain in solution. The modeled L230 dimer structure (magenta ribbon) is fit within the SAXS ab initio envelope (green). Two views rotated 90°. c Surface diagram of the L230 LH domain. Two views rotated 90°. Key tyrosine (yellow) and leucine (pink) residues on the interface are indicated. d Alignment of L230 with human LHs. L230 residues that mediate hydrophobic contacts on the dimerization interface are indicated (arrows). e Size exclusion chromatography with multi-angle static light scattering (SEC-MALS) of L230 LH domain. On the basis of elution time (X-axis) and molar mass (Y-axis), wild-type L230 (blue) forms a dimer, whereas L230 L873D (red) is monomeric. f Enzymatic assay of L230 LH domain proteins using bovine skin collagen as substrate. L873D mutation leads to loss of activity. Results are mean values (±S.D.) from triplicate biological samples. g Enzymatic assay of L230 LH domain proteins using synthetic collagen peptide as substrate. Retention of activity in the mutant suggests that protein integrity is intact. Results are mean values (±S.D.) from triplicate biological samples. p values, two-tailed Student’s t test