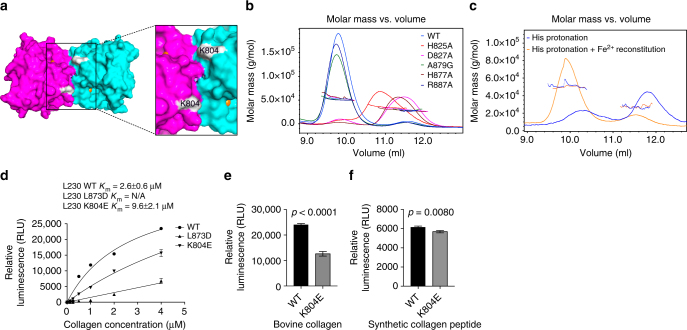
Fig. 3.
A cleft on the protein surface that may be involved in collagen binding. a Surface diagram of the LH domain homodimer. A cleft created by the dimerization interface is flanked by the active sites (inset). Locations of Fe2+ atoms (orange) within each monomer (purple and cyan) are indicated. Location of K804 within the cleft (white). b SEC-MALS of L230 LH domain. On the basis of elution time (X-axis) and molar mass (Y-axis), dimerization is lost following mutation of residues in the amino acid triad (H825, D827, or H877) but not the residue positioned to bind 2-OG (R887) or non-conserved A879. c SEC-MALS of L230 LH domain after Fe2+ removal. Dimerization is lost following histidine protonation to release the Fe2+ (blue line) and restored following Fe2+ reconstitution (orange line). d Enzymatic activity assays of L230 LH domain proteins using different concentrations of bovine skin collagen as substrate. Curves were used to calculate the Km values for wild-type L230 (WT) and L230 containing mutations on the dimerization interface (L873D) or within the cleft (K804E). e, f Enzymatic activity assays of L230 LH domain proteins using bovine skin collagen (e) or synthetic collagen peptide (f) as substrate. Relative to wild-type L230 (WT), K804E exhibited a reduction in activity on collagen, whereas activity in the context of a synthetic peptide was relatively preserved. Results in b–f are mean values (±S.D.) from triplicate biological samples. p values, two-tailed Student’s t test