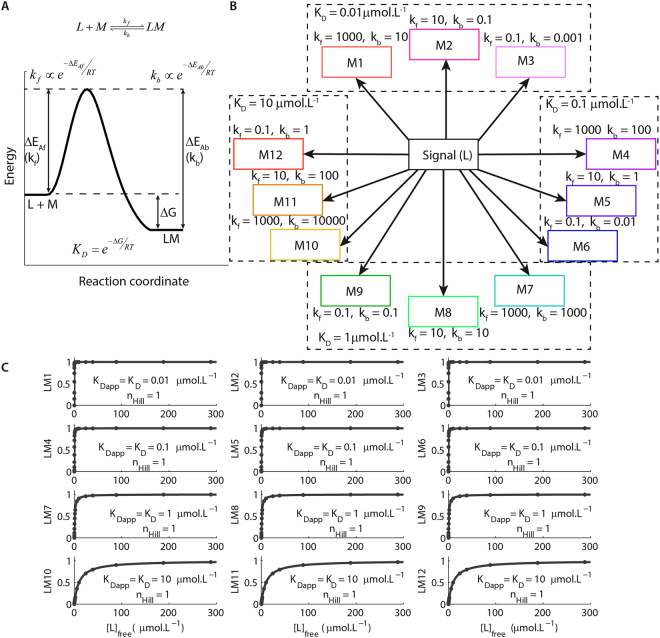
Figure 1.
Thermodynamic and kinetic parameter of chemical reactions. (A) The energy profile for a simple reversible reaction of complex formation. The species L and M are the initial reactants, LM is the complex formed, kf and kb are the rate constants for the association (forward) and dissociation (backward) reactions, respectively. ΔEAf and ΔEAb state for the energy of activation for the forward and backward reaction, respectively, R is the ideal gas constant, and T is the temperature in Kelvin. (B) Diagram of the simulated system, which consists of twelve different molecules (M1-12) interacting with a ligand (L) with different affinities (KDs) and rate constants (kfs are given in µmol.L−1.s−1 and kbs in s−1). (C) Dose-response curves for the formation of the complex LM1-LM12 as functions of [L]free. The KDapps estimated with these curves were set as the control KDs for the formation of LM1-LM12 in our simulations.