Abstract
Protein kinase A (PKA) has been shown to play a role in a plethora of cellular processes ranging from development to memory formation. Its activity is mediated by the catalytic subunits whereby many species express several paralogs. Drosophila encodes three catalytic subunits (PKA-C1–3) and whereas PKA-C1 has been well studied, the functions of the other two subunits were unknown. PKA-C3 is the orthologue of mammalian PRKX/Pkare and they are structurally more closely related to each other than to other catalytic subunits within their species. PRKX is expressed in the nervous system in mice but its function is also unknown. We now show that the loss of PKA-C3 in Drosophila causes copulation defects, though the flies are active and show no defects in other courtship behaviours. This phenotype is specifically due to the loss of PKA-C3 because PKA-C1 cannot replace PKA-C3. PKA-C3 is expressed in two pairs of interneurons that send projections to the ventro-lateral protocerebrum and the mushroom bodies and that synapse onto motor neurons in the ventral nerve cord. Rescue experiments show that expression of PKA-C3 in these interneurons is sufficient for copulation, suggesting a role in relaying information from the sensory system to motor neurons to initiate copulation.
Introduction
Protein kinase A (PKA) is a key regulator in many processes, including cellular growth, embryonic patterning, and learning and memory formation in flies and mammals1–6. PKA has also been connected with sexual behaviour because PKA activity increases when male mice interact with females and mutations in the PKA-regulatory subunit impair courtship suppression in Drosophila, which occurs after males encounter already mated females7–10. PKA is a tetramer of two regulatory and two catalytic subunits, whereby several paralogs for these subunits are found in mammals11 as well as Drosophila. Flies encode three catalytic subunits, PKA-C1–312,13, but functional studies have only been performed with PKA-C1 and the functions of PKA-C3 were unknown. Pka-C3 transcripts have been detected in Northern blot analyses, which revealed expression in pupae and adult heads while weak expression was found in embryos and larvae13. To address a functional redundancy with PKA-C1, PKA-C3 was induced in PKA-C1 mutants by fusing its coding region to the PKA-C1 promoter, however, this did not rescue the phenotypes caused by the loss of PKA-C114. This suggested that the different catalytic subunits have specific functions that cannot be fulfilled by other subtypes. PKA-C3 is evolutionary highly conserved and interestingly it is structurally more closely related to its mammalian orthologue PRKX (also called Pkare) than to PKA-C1 or PKA-C215–17. PRKX is expressed in the developing and adult mouse brain, whereby the pattern during development is restricted to differentiating neurons in the first ganglion, the dorsal root ganglia, and the mantle layer of the telencephalon15,18. PRKX is also found in non-neuronal tissues, such as testes and kidney, and knocking it down in kidney explants resulted in decreased ureteric bud branching19,20. In contrast, neuronal functions of PRKX are also still unknown. We previously found that PKA-C3 activity is inhibited by Swiss-Cheese (SWS) which acts as a non-canonical regulatory subunit that binds to PKA-C3, tethers it to membranes, and inhibits its activity21. SWS specifically interacts with PKA-C3 and does not bind to PKA-C1 or PKA-C2, again supporting unique roles of this unusual PKA complex. However, what these roles are and what consequences the loss of PKA-C3 has for neuronal integrity and function remained unknown.
Results
Loss of PKA-C3 does not affect brain development or integrity
To identify functions of PKA-C3, we first knocked it down pan-neuronally using the Pka-C3NIG.6117R RNAi construct and the Appl-GAL4 driver line. The knockdown was confirmed by performing RT-qPCRs from head homogenates (Supp. Figure 1). The knockdown flies were viable and did not show any overt defects. To determine whether brain development or survival was affected, we performed paraffin head sections of 3d and 30d old flies but neither revealed detectable changes compared to age-matched controls (data not shown). This suggested that the loss of PKA-C3 does not interfere with the development of the brain or with the maintenance of brain integrity during aging. To confirm this, we generated a mutation in PKA-C3. Because no classical allele was available, we created a deletion in the Pka-C3 gene using two piggyBac insertions and FLP/FRT-mediated recombination22. Two alternative transcripts, RA and RB, using different first exons are predicted to be transcribed from the PKA-C3 locus23 and the deletion removes all coding exons of the RA transcript and all coding exons besides the first for RB (Supp. Figure 2A). The first exon of RB encodes 13 amino acids which do not contribute to any known functional domain. The deletion was confirmed by PCR (data not shown) and Western blots that verified that no PKA-C3 protein was detectable in the deletion line (Supp. Figure 2B). Using this mutant, called Pka-C3d, confirmed that the loss of PKA-C3 did not cause defects in the development of the brain or its maintenance during aging (data not shown).
PKA-C3 is required for copulating
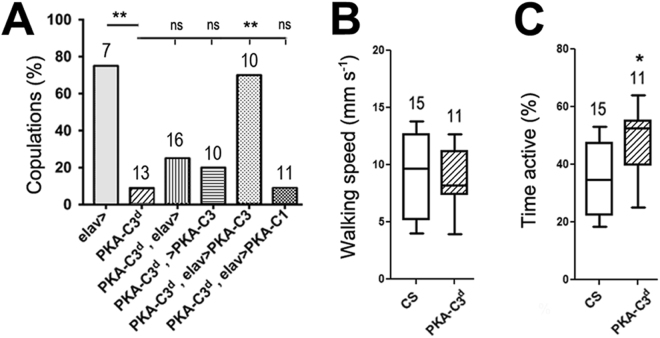
In the creation of Pka-C3d, we noticed that the flies were sterile when homozygous. Whereas Pka-C3d females produced progeny when crossed to wild-type males, this was not the case when we used Pka-C3d males and wild-type females. Determining the courtship behaviour of Pka-C3d males by measuring the time they engaged in stereotypical courtship behaviours, like chasing the wild-type females or extending their wings, we failed to detect a significant difference to wild-type males. (Supp. Figure 3). However, only 9% of the Pka-C3d males attempted to copulate with the females within a 30 min period compared to 71% of the control males (Fig. 1A), showing that the loss of PKA-C3 specifically impaired this component of courtship. Next, we determined whether the copulation deficits are specifically due to the loss of PKA-C3 by performing rescue experiments. As expected, pan-neuronal expression of PKA-C3 via elav-GAL4 in Pka-C3d mutants restored the copulation frequency (Fig. 1A). In contrast, inducing PKA-C1 did not increase copulations and these flies were not significantly different from the PKA-C3d mutant flies (Fig. 1A). This reveals that PKA-C3 has specific functions that cannot be compensated by PKA-C1, although they show strong conservation in their catalytic domain (Supp. Figure 4). In addition, it further supports that PKA-C3 and its mammalian orthologues may form a unique subclass of PKA catalytic subunits with specific functions.
Figure 1.
Pka-C3d mutant males show copulation defects. (A) Percentage of males that copulated with wild-type CS females within the observation period of 30 min. Pka-C3d males performed significantly worse than controls (elav-GAL4). Inducing PKA-C3 with elav-GAL4 restored mating behaviour of Pka-C3d males whereas carrying only the elav-GAL4 driver or the UAS-PKA-C3 construct had no significant effect (ns). Expressing PKA-C1 in Pka-C3d could also not rescue the copulation defects. Flies were 3d old. (B) Walking speed, measured in the Buridan’s paradigm, was not affected in 14d old PKA-C3d mutants compared to wild-type CS. (C) Pka-C3d mutants were more active than wild-type CS controls in the Buridan’s paradigm during the 15-min observation period. Number of individual flies tested is indicated on each bar. A Chi-square test was used to determine significance in A and unpaired student t-tests were used to determine significance in B and C. (B,C) Horizontal lines are medians; boxes are 25 and 75% quartiles; and whiskers, are 10 and 90% quantiles. *p < 0.05, **p < 0.01.
Because the mating defects could be due to reduced activity or locomotion, we measured activity and walking speed of Pka-C3d flies in the Buridan’s paradigm24. In this assay, the flies are recorded while walking back and forth between two inaccessible landmarks and the recordings of this behaviour were used to analyse walking speed and general activity. Analysing the walking speed of Pka-C3d flies demonstrated that they were as fast as wild-type flies (Fig. 1B) and quantifying the time the flies were active showed that Pka-C3d flies actually spend more time moving around than wild-type (Fig. 1C). Together, this shows that the mutant flies are not impaired in their locomotion nor are they inactive. Furthermore, the flies consistently walking back and forth between the landmarks suggested that the loss of PKA-C3 did not interfere with their vision. These findings indicate that the defects in mating are due to deficits in integrating information to orchestrate the appropriate behavioural output, rather than defects in sensory input or motor neuron output.
PKA-C3 is expressed in the ADLI and ICLI interneurons
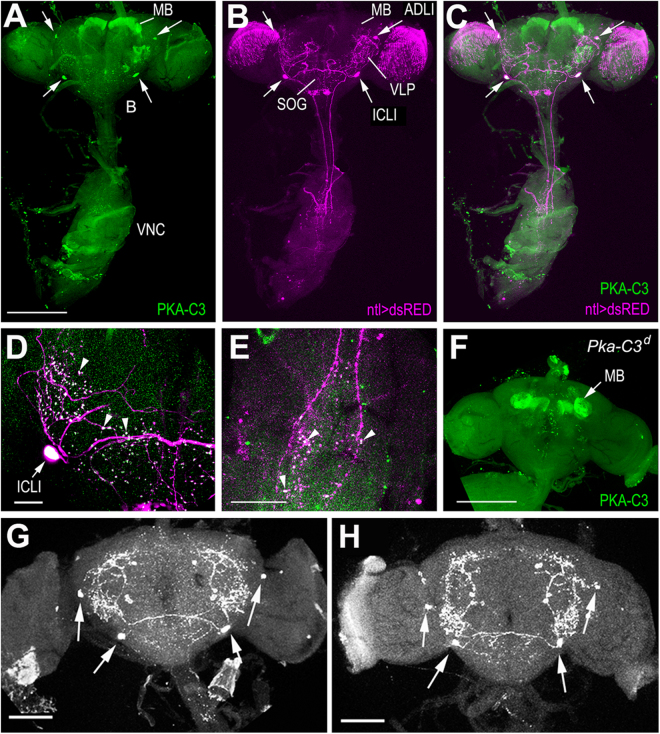
Our pan-neuronal rescue experiments showed that PKA-C3 is required in neurons. To identify whether PKA-C3 is needed in specific neuronal subtypes, we performed immunohistochemistry to determine PKA-C3’s expression pattern with an antibody raised against PKA-C3 in chicken (using the DTKNFDDYPEKDWKPAK peptide highlighted in Supp. Figure 4). Using this antibody on brain/ventral nerve cord preparations, we found strong staining in the mushroom bodies (MB) and in a few large neurons in the protocerebrum (arrows, Fig. 2A). To identify these neurons, we expressed DsRed with various GAL4 lines and performed immunohistochemistry using anti-DsRed and anti-PKA-C3. As shown in Fig. 2C, we found a co-localization of PKA-C3 and DsRed when using natalisin (ntl)-GAL4. Natalisin is a tachykinin-related peptide that is expressed in two pairs of interneurons, the anterior dorso-lateral interneurons (ADLIs) and the inferior contralateral interneurons (ICLIs)25. Furthermore, natalisin has been shown to be involved in mating behaviour, however in contrast to PKA-C3 in both, males and females25. The ADLIs and ICLIs form an extensive neuritic network in the brain that connects the ventro-lateral protocerebrum (VLP), the anterior suboesophageal ganglion (SOG) and the MBs and they send axons into the ventral nerve cord (Fig. 2B and Supp. Figure 5)25. In the brain as well as in the ventral nerve cord, they form varicosities that are positive for Natalisin and interestingly PKA-C3 also predominantly localizes to these varicosities (arrowheads, Fig. 2D,E). To confirm that the immunostaining in these neurons was specific for PKA-C3, we used brain/ventral nerve cord preparations from Pka-C3d mutants. In these preparations the ICLIs and ADLIs were not stained, however the staining of the mushroom bodies persisted (Fig. 2F). A similar result was obtained when using Appl-GAL4 induced knockdown PKA-C3 flies which showed a dramatic reduction of PKA-C3 in the ICLIs and ADLIs while the mushroom bodies were still labelled (Supp. Fig. 6). We therefore concluded that the staining in the MBs is due to cross-reactivity of the antibody with another antigen. To address whether the loss of PKA-C3 positive staining could be due to a loss of the ADLIs and ICLIs in Pka-C3d mutants, we used an antiserum against Natalisin. Because we could easily detect the ADLIs and ICLIs in wild type (Fig. 2G) as well as in Pka-C3d (Fig. 2H) with this antiserum, the loss of PKA-C3 staining was not due to the loss of these cells but to the loss of PKA-C3 in these neurons. This was also confirmed by expressing GFP with ntl-GAL4 in Pka-C3d mutants (data not shown).
Figure 2.
PKA-C3 is expressed in the ADLI and ICLI interneurons. (A) Immunohistochemistry on brain/ventral nerve cord preparations using our anti-PKA-C3 antiserum. Strong PKA-C3-positive staining is detectable in the mushroom bodies (MB) and two pairs of neurons in the protocerebrum (arrows). (B) Expressing DsRed with ntl-GAL4 shows staining in the ADLI (anterior dorso-lateral) and ICLI (inferior contralateral) interneurons that send projections from the brain (B) to the ventral nerve cord (VNC). (C) Overlay of the PKA-C3 and DsRed staining shows expression of PKA-C3 in the ADLIs and ICLIs (arrows). (D) Magnification showing one of the ICLI neurons reveals PKA-C3 positive staining in the cell body (arrow) and the varicosities formed by the ICLI neurites (arrowheads). (E) PKA-C3 can also be detected in the varicosities of the ICLIs in the VNC (arrowheads). (F) Immunohistochemistry with anti-PKA-C3 on a brain whole-mount from a Pka-C3d mutant shows unspecific staining in the MBs whereas the ADLIs and ICLIs are not detectable. (G,H) Loss of PKA-C3 does not interfere with ADLI and ICLI survival because we can detect them in wild-type flies (G) as well as in PKA-C3d flies (H) using an antiserum against Natalisin. The arrows point to the cell bodies of the ADLIs and ICLIs. SOG; = suboesophageal ganglion, VLP; = ventro-lateral protocerebrum. Scale bar in A, F = 200 µm, in D, E = 50 µm, in G, H = 100 µm.
Loss of PKA-C3 in the ADLIs and ICLIs is sufficient to restore copulation
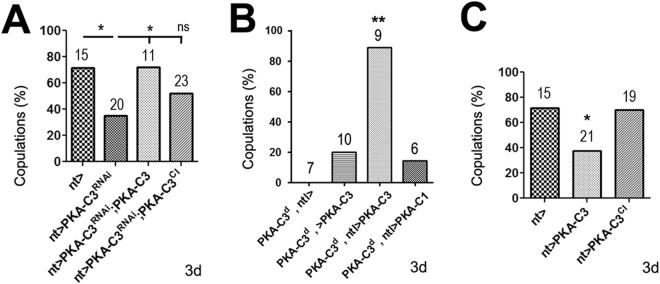
The immunohistochemistry reveals that PKA-C3 is expressed in the ADLI and ICLI interneurons and due to their morphology, these neurons could play an important role in integrating information that then controls behavioural output. We therefore first addressed whether the loss of PKA-C3 in these neurons is sufficient to induce the copulation deficits by expressing the Pka-C3NIG.6117R RNAi construct with the ntl-GAL4 driver. Analyzing courtship behaviour, we found a significant reduction in copulation events when ntl-GAL4 induced PKA-C3 knockdown males were presented with wild-type females (Fig. 3A). This confirms that PKA-C3 is necessary in these two pairs of neurons for normal mating. Next, we investigated whether expression of PKA-C3 in the ADLIs and ICLIs is sufficient to rescue the phenotypes after the loss of PKA-C3. As shown in Fig. 3A,B, induction of PKA-C3 via ntl-GAL4 was sufficient to restore mating behaviour of PKA-C3 knockdown mutant males. We also addressed whether the catalytic activity of PKA-C3 is required for its function by using a constitutively inactive form of PKA-C3 in which aspartate 397 in the catalytic loop is replaced by alanine (see Supp. Figure 3). Although this construct is expressed at higher levels than our normal PKA-C3 construct, it did not increase PKA activity whereas PKA-C3 did (Supp. Figure 7), suggesting that it is indeed a catalytically inactive form. In contrast to PKA-C3, the inactive PKA-C3D397A did not rescue the copulation defects (Fig. 3A), showing that the PKA catalytic activity is required for the function of PKA-C3. As expected, expression of PKA-C3 with ntl-GAL4 also restored the mating behaviour of Pka-C3d mutant males. As shown with the pan-neuronal expression, induction of PKA-C1 with ntl-GAL4 did not rescue the mating defects (Fig. 3B), again confirming the specificity of PKA-C3. Lastly, we tested whether overexpression of PKA-C3 in Natalisn neurons induces mating phenotypes. As shown in Fig. 3C, induction of wild-type PKA-C3 did reduce the copulation events, whereas the inactive form did not. This suggests that the levels of PKA-C3 have to be tightly regulated and that the catalytic activity is required for both, restoring the normal function and causing overexpression phenotypes.
Figure 3.
Expression of PKA-C3 is required in the ADLIs and ICLIs. (A) Knocking down PKA-C3 with ntl-GAL4 and PKA-C3NIG.6117R causes reduces copulations of 3old males. Expressing wild-type PKA-C3 with ntl-GAL4 in the knockdown flies restores copulations whereas expression of the constitutively inactive PKA-C3 (PKA-C3CI) does not. (B) Expressing PKA-C3 with ntl-GAL4 in Pka-C3d mutants is sufficient to restore mating while expression of PKA-C1 has no effect. (C) ntl-GAL4 driven expression of PKA-C3 in the wild-type background reduces copulations while additional expression of PKA-C3CI does not. Statistical analysis was done with one way Anova and Chi-square tests. Number of individuals tested and the SEMs are indicated. *p < 0.05, **p < 0.01, ns not significant.
The ADLIs and ICLIs connect onto motor neurons
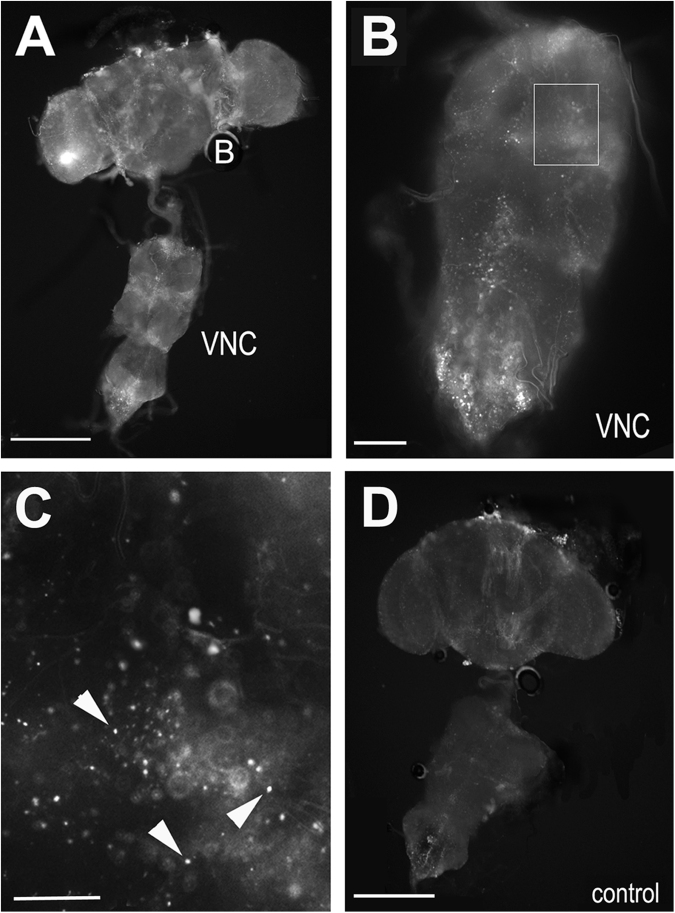
The ADLIs and ICLIs are well-suited to relay information from the sensory system and the brain to motor neurons, which are localized in the ventral nerve cord, thereby controlling behaviour. To determine whether these neurons do indeed connect to motor neurons, we used a GFP reconstitution system26. For this, UAS-CD4-spGFP1–10 was induced in the ICLIs and ADLIs with ntl-GAL4 while lexAop-CD4-spGFP11 was expressed with a vGlut-lexA driver, which due to motor neurons being glutamatergic is expressed in motor neurons27. As shown in Fig. 4A,B, we clearly detected GFP in the brain and ventral nerve cord in these flies, showing that these interneurons do form connections with glutamatergic neurons in the ventral nerve cord. A higher magnification revealed that GFP is detectable in a punctuate pattern along the midline of the ventral nerve cord, an area in which we also observed the ntl-GAL4 > DsRed and PKA-C3-positive varicosities (Fig. 4C, compare to Fig. 2E). In contrast, this was not the case in control flies that expressed only one part of GFP, UAS-CD4-spGFP1–10, via ntl-GAL4 (Fig. 4D). These findings supports our hypothesis that PKA-C3 is localized at synapses that connect the ADLIs and/or ICLIs with motor neurons.
Figure 4.
GFP reconstitution experiments show connections of the ICLIs to glutamatergic neurons in the ventral nerve cord. (A,B) Reconstituting GFP by expressing UAS-CD4-spGFP1-10 with ntl-GAL4 and lexAop-CD4-spGFP11 with GMR52D11 results in a punctuate staining in the brain (B) and ventral nerve cord (VCN). (C) Magnification of the area boxed in (B) shows reconstitution in puncta, possibly synapses (arrowheads). (D) A ntl-GAL4 > UAS-CD4-spGFP1-10 control fly does not show this pattern. Scale bars in A, D = 200 µm, in B = 50 µm, in C = 15 µm.
Discussion
As in other species, PKA complexes in Drosophila can contain different catalytic subunits but while the C1 subunit has been extensively studied, so far nothing was known about the specific distribution or function of the C3 subunit. Whereas the loss of PKA-C1 is lethal during development3,28, we show that the deletion of PKA-C3 is viable and exhibits no overt anatomical phenotypes. However, PKA-C3 mutants show behavioural deficits in mating, specifically a reduction in copulations. Due to their structural differences, we anticipated that PKA-C3 has specific functions not shared by PKA-C1 and indeed PKA-C1 expression could not compensate for the loss of PKA-C3. In addition, these two catalytic subunits appear to be present in different neuronal subpopulations in the adult brain. In agreement with its role in learning and memory, PKA-C1 is predominantly expressed in the mushroom bodies29–31 whereas we found PKA-C3 to be predominantly expressed in the ADLIs and ICLIs. That PKA-C3 is required in these interneurons was confirmed by our knockdown experiments which revealed that loss of PKA-C3 only in these four neurons is sufficient to induce the mating phenotypes. In addition, expression of PKA-C3 specifically in these neurons could completely rescue the copulation defects, showing that induction of PKA-C3 only in these neurons is sufficient for normal mating behaviour. Furthermore, using a constitutively inactive form of PKA-C3, we show that its function in copulation behaviour is indeed dependent on the kinase activity. Previous work by Jiang and colleagues demonstrated that the ICLIs and ADLIs play a critical role in mating behaviour and that successful mating requires Natalisin which is expressed in these neurons25. Because the ICLIs and ADLIs are present in our mutant, the mating defects are not caused by the loss of these neurons but by the loss of PKA-C3 in these neurons. Furthermore, using an antiserum against Natalisin, we could not detect changes in the levels or localization of Natalisin in the mutant, suggesting that PKA-C3 does not impair copulation via interfering with Natalisin signaling. Therefore, PKA-C3 seems to affect other pathways, however so far no targets of PKA-C3 are known. Identifying these targets could therefore provide more insights into the regulation of mating behaviour and the specific function of the ADLIs and ICLIs in this behaviour. We assume that PKA-C3 may be involved in the process of integrating information to achieve an appropriate motor neuron output. This is based on our findings that Pka-C3d mutants can track the landmarks in the Buridan’s paradigm and can follow and court females, showing that they not only receive visual input but can also coordinate and execute locomotion output for these behaviours. The ADLIs and ICLIs connect the VLP, which receives visual, olfactory and gustatory input32,33, with the mushroom bodies, which are crucial for learning and memory34, and the ventral nerve cord. Because mutations that affect olfaction or taste show a reduction in the courtship index (CI)35 and the CI is not decreased in our mutant, we assume that none of the sensory inputs required for initiating and maintaining courtship is affected. However, it has been suggested that copulation also requires a feedback from the females35 and it is possible that Pka-C3d mutants are unable to interpret or integrate these feedback responses to then initiate copulation. However, a better understanding of the mechanisms regulating these feedback responses is needed to address this issue and the possible role of PKA-C3 in this process.
Methods
Drosophila stocks
CantonS, elav-GAL4, UAS-PKA-C1, UAS-DsRed, GMR52D11-lexA and P{UAS-CD4-spGFP1–10}3, P{lexAop-CD4-spGFP11}3 were obtained from the Bloomington Stock Center. Pka-C3NIG.6117R was obtained from the National Institute of Genetics (NIG-Fly), Japan. natalisin-GAL4 was kindly provided by Y. Park and Y-J. Kim and is described in25. PBacf07226 and PBacPka-C3f00695 were obtained from the Exelixis Collection at the Harvard Medical School. UAS-PKA-C3 is described in21. The constitutively inactive PKA-C3 construct was generated by replacing aspartate 397 (D397, see Supp. Figure 3) with alanine by site–directed mutagenesis using the site-directed mutagenesis kit from clontech. D397 is part of the highly conserved YRDLKPEN core sequence of the catalytic loop and mutations in the conserved aspartate have been shown to abolish or dramatically reduce the catalytic activity36–38. The construct was tagged with HA. Flies were maintained on standard fly food under a 12:12 h light:dark cycle. Stocks were maintained at 18 °C while crosses and aging flies were maintained at 25 °C. The age of the flies in each experiment is indicated in the figures and figure legends.
Immunohistochemistry
Brains were dissected in ice-cold PBS and transferred to 4% PFA in PBS. They were then fixed for 20 minutes at room temperature (RT) and washed four times with PBS/0.5% Triton (PBS-T) for 13 min each before blocking with 2% BSA in PBS-T for 2 hours at RT. Anti-RFP (Millipore AB3216) was used at 1:250 overnight at 4 C. Brains were then washed three times, 20 min each at RT and the secondary antibody applied (anti-rabbit-Cy2, Jackson ImmunoResearch) at 1:250 for 2 hours at RT. Brains were washed three times for 20 minutes with PBS-T and mounted in Glycergel for confocal imaging. For anti-PKA-C3 and Natalisin detection, brains were dissected in ice-cold PBS and fixed in 4% PFA for 45 min at room temperature. After washing four times for 13 min each with PBS-T, they were blocked with 2% BSA in PBS-T for 90 min with changing the blocking solution once in between. Anti-PKA-C3, raised against the DTKNFDDYPEKDWKPAK peptide in chicken (Aves Labs, Oregon), was used at 1:500 and rabbit anti-DmNTL4 (ref.25, kindly provided by Y-J. Kim, Gwangju Institute of Science and Technology, South Korea) at 1:1000 for 2 hours at RT, followed by incubation over night at 4 °C. Brains were then washed four times 13 min at RT and the secondary antibody (anti-chicken-Alexa488, Molecular Probes/Life Technologies) applied at 1:250 for 2 hours at RT. Brains were washed three times for 20 minutes with PBS-T and then mounted for confocal imaging using an Olympus FluoView 300 laser scanning confocal head mounted on an Olympus BX51 microscope.
Buridan’s Paradigm
Flies with their wings cut (one day before testing) were analysed in a cylindrical, brightly illuminated arena that presented two opposing, inaccessible black stripes as described in24. Activity reflects the time spent walking during the 15-min observation time in %. The mean walking speed was averaged over the whole observation time in mm/s. GraphPad Prism and Shapiro-Wilk tests were used to test for normal distribution and unpaired t-tests for significance.
Mating assays
Virgin wild type CantonS females were collected and kept on food vials for 3 days. Using an aspirator, single females were transferred to the courtship chamber (6 mm height × 10 mm diameter, kindly provided by C. Helfrich-Förster, University Würzburg) and an experimental male added that was also collected shortly after eclosion and aged for 3 days. Video recordings were done for 30 min using a Nikon Coolpix p500 camera. Successful mating occurred when the males copulated during the 30 minute recording period as described in25. The courtship assays were performed following the methods described in39. Chi square tests were used to determine significance. The Courtship Index (CI) was calculated by determining the time the males performed any type of courtship-associated behaviour divided by the total time of recording (10 min) as described in40. To determine a significant difference in the CI, GraphPad Prism and Mann-Whitney tests were used.
Electronic supplementary material
Acknowledgements
We thank all colleagues who provided fly stocks. We are also grateful to Dr. Stephanie Kaech-Petrie for help with confocal imaging and to Dr. Burkhard Poeck for critical reading of the manuscript. This work was supported by a grant (NS047663) to D.K. from NIH/NINDS. E.S. was supported by a training grant from the NIH (5T32AG023477) and M.C. by a grant from the Collins Foundation. S.K. was supported by a Ph.D. scholarship from the Stipendienstiftung Rheinland-Pfalz to S.K., and S.K. and R.S. by funding by German Science Foundation (DFG; STR590/6–1). We also received support by the NIH P30NS061800 grant to the OHSU imaging facility.
Author Contributions
M.C., E.S., J.S.W., and S.K. performed experiments; R.S. and D.K. designed the experiments and D.K. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20697-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lane ME, Kalderon D. RNA localization along the anteroposterior axis of the Drosophila oocyte requires PKA-mediated signal transduction to direct normal microtubule organization. Genes & Development. 1994;8:2986–2995. doi: 10.1101/gad.8.24.2986. [DOI] [PubMed] [Google Scholar]
- 2.Mayford M, Abel T, Kandel ER. Transgenic approaches to cognition. Current Opinion in Neurobiology. 1995;5:141–148. doi: 10.1016/0959-4388(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 3.Pan D, Rubin GM. cAMP-dependent protein kinase and hedgehog act antagonistically in regulating decapentaplegic transcription in Drosophila imaginal discs. Cell. 1995;80:543–552. doi: 10.1016/0092-8674(95)90508-1. [DOI] [PubMed] [Google Scholar]
- 4.Roman, G. & Davis, R. L. Vol. 23, 571–581 (2001). [DOI] [PubMed]
- 5.Skoulakis EMC, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of PKA and its role in learning and memory. Abstracts of papers presented at the 1993 Cold Spring Harbor meeting on Neurobiology of Drosophila October 6–10. 1993;1993:200. [Google Scholar]
- 6.Walsh DA, Van Patten SM. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB Journal. 1994;8:1227–1236. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- 7.Goto A, et al. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci USA. 2015;112:6718–6723. doi: 10.1073/pnas.1507121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHenry JA, Bell GA, Parrish BP, Hull EM. Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats. Behavioral neuroscience. 2012;126:523–529. doi: 10.1037/a0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Dell KM, Jamieson D, Goodwin SF, Kaiser K. Abnormal courtship conditioning in males mutant for the RI regulatory subunit of Drosophila protein kinase A. Journal of neurogenetics. 1999;13:105–118. doi: 10.3109/01677069909083469. [DOI] [PubMed] [Google Scholar]
- 10.Winbush A, et al. Identification of Gene Expression Changes Associated With Long-Term Memory of Courtship Rejection in Drosophila Males. G3: Genes|Genomes|Genetics. 2012;2:1437–1445. doi: 10.1534/g3.112.004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beebe SJ. The cAMP-dependent protein kinases and cAMP signal transduction. Seminars in cancer biology. 1994;5:285–294. [PubMed] [Google Scholar]
- 12.Inoue H, Yoshioka T. Purification of a regulatory subunit of type II cAMP-dependent protein kinase from Drosophila heads. Biochemical and Biophysical Research Communications. 1997;235:223–226. doi: 10.1006/bbrc.1997.6764. [DOI] [PubMed] [Google Scholar]
- 13.Kalderon D, Rubin GM. Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes & Development. 1988;2:1539–1556. doi: 10.1101/gad.2.12a.1539. [DOI] [PubMed] [Google Scholar]
- 14.Melendez A, Li W, Kalderon D. Activity, expression and function of a second Drosophila protein kinase A catalytic subunit gene. Genetics. 1995;141:1507–1520. doi: 10.1093/genetics/141.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaschke RJ, Monaghan AP, Bock D, Rappold GA. A novel murine PKA-related protein kinase involved in neuronal differentiation. Genomics. 2000;64:187–194. doi: 10.1006/geno.2000.6116. [DOI] [PubMed] [Google Scholar]
- 16.Klink A, et al. The human protein kinase gene PKX1 on Xp22.3 displays Xp/Yp homology and is a site of chromosomal instability. Hum Mol Genet. 1995;4:869–878. doi: 10.1093/hmg/4.5.869. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Li Q, Alberts I, Li X. PRKX, a Novel cAMP-Dependent Protein Kinase Member, Plays an Important Role in Development. Journal of cellular biochemistry. 2016;117:566–573. doi: 10.1002/jcb.25304. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Yu ZX, Kotin RM. Profiles of PrKX expression in developmental mouse embryo and human tissues. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2005;53:1003–1009. doi: 10.1369/jhc.4A6568.2005. [DOI] [PubMed] [Google Scholar]
- 19.Li X, et al. Protein kinase-X interacts with Pin-1 and Polycystin-1 during mouse kidney development. Kidney international. 2009;76:54–62. doi: 10.1038/ki.2009.95. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Iomini C, Hyink D, Wilson PD. PRKX critically regulates endothelial cell proliferation, migration, and vascular-like structure formation. Developmental biology. 2011;356:475–485. doi: 10.1016/j.ydbio.2011.05.673. [DOI] [PubMed] [Google Scholar]
- 21.Bettencourt da Cruz A, Wentzell J, Kretzschmar D. Swiss Cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3. Journal of Neuroscience. 2008;28:10885–10892. doi: 10.1523/JNEUROSCI.3015-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golic KG, Golic MM. Engineering the Drosophila genome: chromosome rearrangements by design. Genetics. 1996;144:1693–1711. doi: 10.1093/genetics/144.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gramates LS, et al. FlyBase at 25: looking to the future. Nucleic acids research. 2017;45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Götz, K. G. Visual guidance in Drosophila. In Development and Neurobiology of Drosophila Plenum Press New York, London, Washington, Boston, 391–407 (1980).
- 25.Jiang H, et al. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc Natl Acad Sci USA. 2013;110:E3526–3534. doi: 10.1073/pnas.1310676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene expression patterns: GEP. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Lane ME, Kalderon D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes & Development. 1993;7:1229–1243. doi: 10.1101/gad.7.7a.1229. [DOI] [PubMed] [Google Scholar]
- 29.Skoulakis EMC, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-T. [DOI] [PubMed] [Google Scholar]
- 30.Crittenden JR, Skoulakis EMC, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learning and Memory. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Tully T, Kalderon D. Effects of a conditional Drosophila PKA mutant on olfactory learning and memory. Learning and Memory. 1996;2:320–333. doi: 10.1101/lm.2.6.320. [DOI] [PubMed] [Google Scholar]
- 32.Ebbs ML, Amrein H. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflugers Archiv: European journal of physiology. 2007;454:735–747. doi: 10.1007/s00424-007-0246-y. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka NK, Endo K, Ito K. Organization of antennal lobe-associated neurons in adult Drosophila melanogaster brain. The Journal of comparative neurology. 2012;520:4067–4130. doi: 10.1002/cne.23142. [DOI] [PubMed] [Google Scholar]
- 34.Heisenberg M. Learning & memory (Cold Spring Harbor, N.Y.) 1998. What do the mushroom bodies do for the insect brain? an introduction; pp. 1–10. [PMC free article] [PubMed] [Google Scholar]
- 35.Krstic D, Boll W, Noll M. Sensory integration regulating male courtship behavior in Drosophila. PloS one. 2009;4:e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe T, et al. Site-directed mutagenesis of the active site of diacylglycerol kinase alpha: calcium and phosphatidylserine stimulate enzyme activity via distinct mechanisms. Biochemical Journal. 2003;375:673–680. doi: 10.1042/bj20031052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9:576–596. doi: 10.1096/fasebj.9.8.7768349. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends in biochemical sciences. 2011;36:65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols, C. D., Becnel, J. & Pandey, U. B. Methods to assay Drosophila behavior. Journal of visualized experiments: JoVE, 10.3791/3795 (2012). [DOI] [PMC free article] [PubMed]
- 40.Gailey DA, Jackson FR, Siegel RW. Male courtship in Drosophila: the conditioned response to immature males and its genetic control. Genetics. 1982;102:771–782. doi: 10.1093/genetics/102.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.