Abstract
Patient-ventilator asynchrony (PVA) are a common phenomenon affecting all patients receiving long-term domiciliary ventilation. The interruption of synchrony between the patient and the ventilator has been reported to be a possible cause of nocturnal sleep disruption leaking to arousals, awakenings and reduced periods of stage 3 and REM sleep overnight. The cause of PVA is multi-factorial driven frequently by leak at the interface, changing respiratory breathing patterns and changes in sleep stages. It currently remains unclear as to whether the PVA is the cause of sleep fragmentation or if PVA is purely a marker of unsuccessful ventilatory control, patient discomfort and underlying sleep disruption.
Keywords: Patient-ventilator asynchrony (PVA), sleep disruption, non-invasive ventilation (NIV), leak
Introduction
Sleep disordered breathing is common in patients with chronic respiratory failure. Respiratory failure results from an imbalance between the loads placed upon the respiratory system and its capacity to compensate for that load. To balance the load on the respiratory system in patients with respiratory disease, neural respiratory drive is elevated to activate and recruit muscle to increase the operational capacity from the respiratory muscle pump. Overnight, the natural fall in neural respiratory drive during sleep, particularly in rapid eye movement (REM) sleep exacerbates the respiratory muscle load-capacity imbalance. This occurs in combination with muscle hypotonia of the upper airway musculature and a blunted hypercapnic ventilatory response predisposing to alveolar hypoventilation and respiratory failure (1,2). Sleep disordered breathing frequently develops as an early sign of underlying respiratory failure leading to sleep fragmentation as the patient awakes to augment neural respiratory drive and daytime somnolence (3,4).
Nocturnal non-invasive ventilation (NIV) reduces the work of breathing, alleviates upper airways obstruction and assists the respiratory muscle pump to improve alveolar hypoventilation and gas exchange. As such, it is the treatment of choice in managing patients with chronic respiratory failure. Correcting sleep disordered breathing with NIV may therefore be expected to improve both nocturnal gas exchange and sleep quality by ameliorating the respiratory disturbance index. However, significant sleep disruption has been reported during the use of both invasive mechanical and NIV which may adversely impact on patients’ health, wellbeing and adherence to therapy (5-7).
Patient-ventilator asynchrony (PVA) describes the poor interaction between the patient and the ventilator and is the consequence of the respiratory muscle activity of the patient being opposed to the action of the ventilator. A mismatch between the breaths demanded by the patient and those delivered by the ventilator has been proposed as a mechanism of leading to increased sleep disruption in patients requiring nocturnal NIV.
PVA during NIV
PVA is observed in all patients receiving NIV with a majority of patients experiencing >10% breaths affected by this phenomenon (8). A recent study in patients with amyotrophic lateral sclerosis receiving long term domiciliary NIV suggested that PVA affected up to 5% of the total sleep time (9).
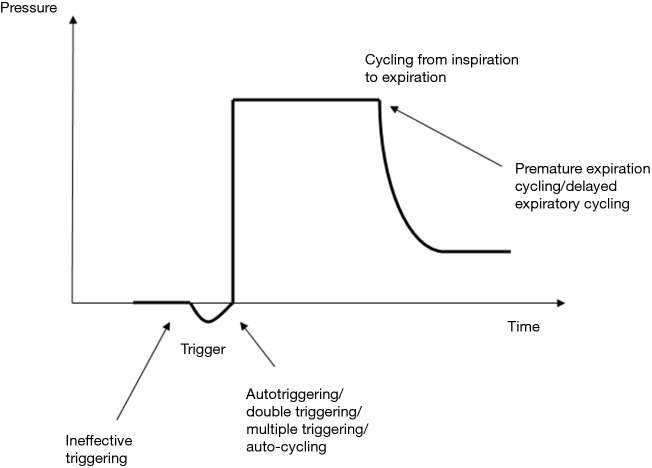
PVA can be characterised into those that interrupt the triggering of the ventilator ‘triggering asynchrony’ and those that affect the cycling of the ventilator from inspiration to exhalation ‘cycling asynchrony’. This can be observed most clearly when examining the ventilator delivered breath waveform in more detail (Figure 1).
Figure 1.
Demonstrating the different types of patient-ventilator asynchrony that can interrupt the harmony between the patient demands and ventilator delivered breaths.
Triggering asynchrony
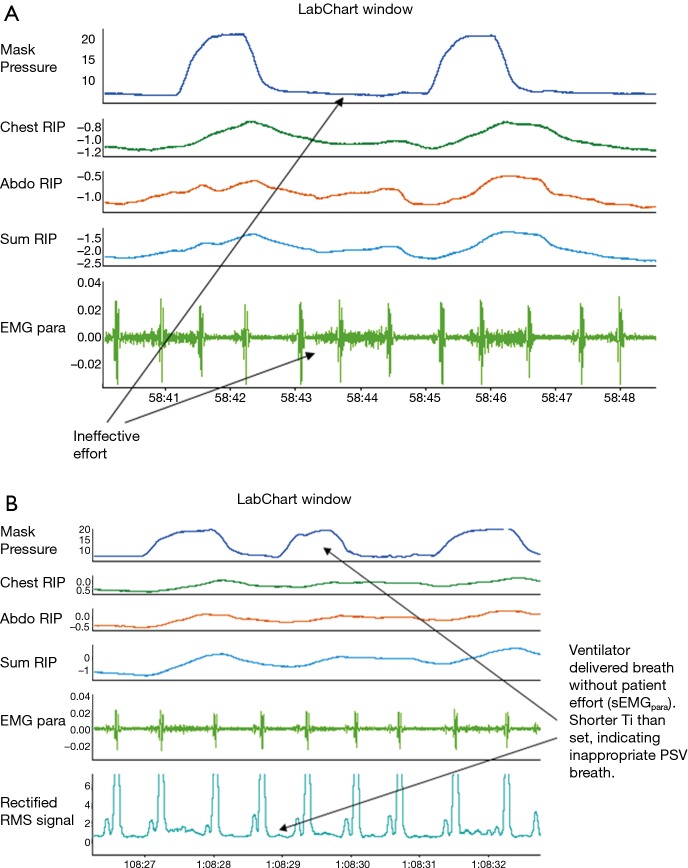
Triggering asynchrony are the most common type of asynchrony observed affecting almost a quarter of all breaths with ineffective triggering accounting for the majority (16% of breaths) (8). Ineffective triggering occurs when the patient attempts to exhibit inspiratory effort demanding a breath without a corresponding breath being delivered by the ventilator (Figure 2). This has the opposite desired effect for a patient in respiratory failure as it increases the work of breathing performed by the patient without any support or unloading of the respiratory muscles by the ventilator.
Figure 2.
An example of (A) ‘ineffective triggering’ and (B) ‘autotriggering’ asynchrony. Chest RIP, chest wall respiratory inductance plethysmography; Abdo RIP, abdominal wall respiratory inductance plethysmography; Sum RIP, sum of both the chest wall and abdominal inductance plethysmography bands; EMGpara, surface second intercostal parasternal electromyography signal reflecting patient neural inspiratory drive; Rectified RMS Signal, rectified root mean square of the parasternal electromyography signal to better characterise onset and termination of neural inspiratory time; Ti, inspiratory time; PSV, pressure support ventilation delivered breath.
By contrast, autotriggering occurs when an inappropriate breath is delivered by the ventilator that has not been requested by the patient. This phenomenon can result in the delivery of a single inappropriate breath ‘autotriggering’ or the patient may request a single breath and the ventilator will deliver two breaths in quick succession ‘double triggering’ or more than two breaths in quick succession ‘multiple triggering’.
Cycling asynchrony
Cycling asynchrony have been reported as less common than triggering asynchrony affecting approximately 5% of breaths in patients receiving long-term home mechanical ventilation (HMV) (8). However, in a study of patients requiring NIV for acute respiratory failure, cycling asynchrony were more prominent with delayed expiratory cycling the most frequent asynchrony detected, affecting 23% of patients studied (10).
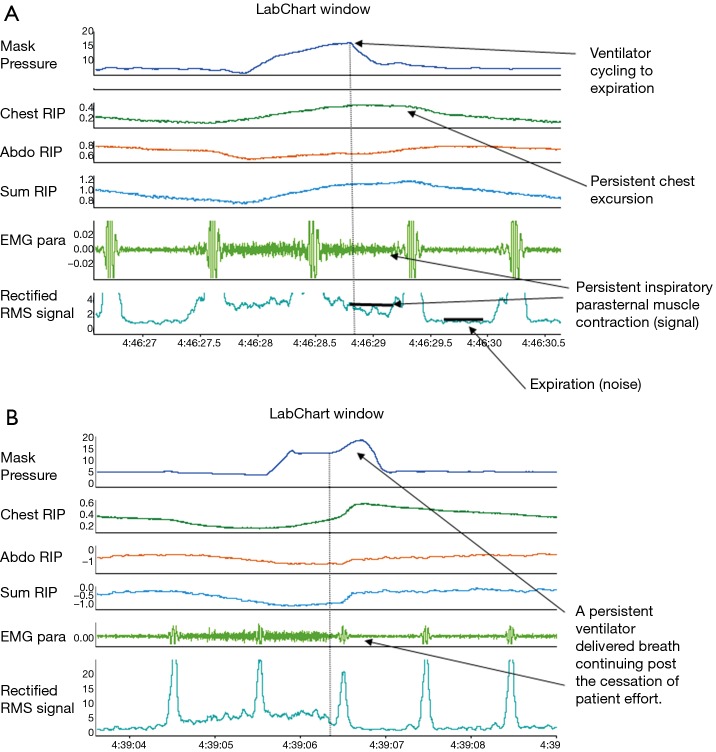
Cycling asynchrony occur when there is a mismatch between the inspiratory time that the patient demands termed ‘neural inspiratory time’ and the inspiratory time provided by the ventilator. Either the patient may wish to breathe in for an extended period of time and the ventilator inappropriately cycles into expiration early ‘premature expiratory cycling’ or the patient may wish to exhale and the ventilator inappropriately continues to deliver an inspiratory breath ‘delayed expiratory cycling’ (Figure 3).
Figure 3.
An example of (A) ‘premature expiratory cycling’ and (B) ‘delayed expiratory cycling’ asynchrony. Chest RIP, chest wall respiratory inductance plethysmography; Abdo RIP, abdominal wall respiratory inductance plethysmography; Sum RIP, sum of both the chest wall and abdominal inductance plethysmography bands; EMGpara, surface second intercostal parasternal electromyography signal reflecting patient neural inspiratory drive; Rectified RMS Signal, rectified root mean square of the parasternal electromyography signal to better characterise onset and termination of neural inspiratory time.
Autocycling occurs when the ventilator is in ‘free fall’ and continues to cycle between inspiration and expiration delivering multiple breaths that are completely out of synchrony with any inspiratory effort made by the patient.
PVA and sleep disruption
In a study assessing patient experiences of mechanical ventilation, 35% retrospectively described significant sleep disruption (11). Thirty percent reported symptoms of ‘agony/panic’ during mechanical ventilation that was associated with difficulties in synchronising the patients breathing to the ventilator (11). This has led to the suggestion that patient ventilator asynchrony may be directly related with arousals and awakenings resulting in persistent sleep disruption. Previous studies that examined patients with chronic respiratory failure receiving HMV, demonstrated a relationship between increased PVA and poor sleep quality, defined as greater sleep fragmentation with reduced stage 3 and REM sleep (5,12,13). Research by Fanfulla et al., also demonstrated an association between the number of ineffective efforts observed and the time spent overnight in REM sleep in patients with neuromuscular disease receiving NIV, suggesting that ineffective effort asynchrony may inhibit REM sleep. Guo et al., identified similar findings in 20 patients with obesity hypoventilation syndrome receiving nocturnal NIV, where PVA was associated with a reduction in stage 3 and REM sleep (12). PVA was also associated with arousals predominantly in lighter stage 1 & 2 sleep which may be related to enhanced neural respiratory drive to the respiratory muscles in light sleep facilitating opposition to the ventilator delivered breaths (12).
Over time with increased experience of NIV there have been improvements in ventilatory strategy, most notably a move to higher levels of pressure support and rates of ventilator delivered breaths to unload respiratory muscles and control carbon dioxide levels. Newer modes of ventilation have also been adopted in an attempt to enhance ventilation, patient comfort and patient ventilator synchronisation. Bosma et al. compared proportional assist ventilation (which delivers a partial ventilatory support controlled by the patient’s demand augmenting the flow and volume delivered to the patient) to pressure support ventilation in patients weaning from mechanical ventilation. They reported that proportional assist ventilation improved sleep disruption as a consequence of improved patient ventilator interaction and a reduction in PVA (6).
Despite improvements in the delivery of ventilation, the levels of PVA in patients receiving domiciliary NIV remain high (8,9). The clinical benefits of nocturnal NIV to patients in chronic respiratory failure remains clear and from experience, the majority of patients adhere to treatment without intolerable sleep disruption. Furthermore, recent studies have been challenging the earlier literature suggesting that a direct association between PVA and sleep disruption is far from clear (9). Changes between sleep stages overnight in patients with sleep disruption may impact on levels of neural respiratory drive and therefore disturb the interaction of the patient with the ventilator. Deleterious changes in gas exchange overnight may also lead to hypoxia induced arousals from events such as residual obstructive apnoea or hypercapnic blunting of neural respiratory drive. Also, obstructive apnoea may directly lead to ineffective effort asynchrony interfering with the ventilator detection of patient inspiratory effort due to the cessation of flow. Therefore, whether PVA occurs as a marker of underlying poor sleep disruption or is the driver of the sleep disruption is yet to be fully characterised.
PVA and leak
Leak is a further complexity that accompanies the non-invasive interface used to deliver ventilation and impacts on the interaction between the patient and ventilator. Leaks are intentional occurring via the exhalation valve to facilitate carbon dioxide clearance and unintentional leak occurring elsewhere in the ventilator circuit. Unintentional leak interferes with the ventilator detection of patient ventilatory efforts leading to both triggering asynchrony and delayed expiratory cycling asynchrony (13). These asynchrony were correlated with increased arousal and awakenings (13). However, more recent studies focusing on patients with amyotrophic lateral sclerosis in whom unintentional leak is a common phenomenon due to facial muscle weakness did not demonstrate a significant impact on sleep architecture (9). Unintentional leak also increases airflow across the skin and is associated with a sudden noise, which may itself have a deleterious impact on sleep quality. Identifying if PVA associated with leak is the driver of sleep disruption or just a consequence of external factors is challenging.
Conclusions
In summary, PVA are frequent during domiciliary NIV. These asynchronies may impact on ventilator efficiency, respiratory muscle loading, patient comfort and sleep disruption, although the overall importance of PVA in NIV is becoming less certain. The current literature is conflicted in the evidence supporting PVA as a direct cause of significant sleep disruption. Similarly, recent studies have observed considerable PVA without any significant impact on gas exchange, challenging earlier findings (8,9,12). The relationship between sleep disruption resulting from PVA, leaks, gas exchange, and clinical outcomes such as adherence to therapy, health-related quality of life measures and survival require further evaluation.
Acknowledgements
The author acknowledges the lane fox respiratory research unit and King's College London University.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Becker HF, Piper AJ, Flynn WE, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med 1999;159:112-8. 10.1164/ajrccm.159.1.9803037 [DOI] [PubMed] [Google Scholar]
- 2.Elliott MW. Non-invasive ventilation during sleep: time to define new tools in the systematic evaluation of the technique. Thorax 2011;66:82-4. 10.1136/thx.2010.142117 [DOI] [PubMed] [Google Scholar]
- 3.Ragette R, Mellies U, Schwake C, et al. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax 2002;57:724-8. 10.1136/thorax.57.8.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punjabi NM, O'Hearn DJ, Neubauer DN, et al. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med 1999;159:1703-9. 10.1164/ajrccm.159.6.9808095 [DOI] [PubMed] [Google Scholar]
- 5.Fanfulla F, Delmastro M, Berardinelli A, et al. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med 2005;172:619-24. 10.1164/rccm.200406-694OC [DOI] [PubMed] [Google Scholar]
- 6.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007;35:1048-54. 10.1097/01.CCM.0000260055.64235.7C [DOI] [PubMed] [Google Scholar]
- 7.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med 2002;166:1423-9. 10.1164/rccm.200209-999OC [DOI] [PubMed] [Google Scholar]
- 8.Ramsay M, Mandal S, Suh ES, et al. Parasternal electromyography to determine the relationship between patient-ventilator asynchrony and nocturnal gas exchange during home mechanical ventilation set-up. Thorax 2015;70:946-52. 10.1136/thoraxjnl-2015-206944 [DOI] [PubMed] [Google Scholar]
- 9.Vrijsen B, Testelmans D, Belge C, et al. Patient-ventilator asynchrony, leaks and sleep in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2016;17:343-50. 10.3109/21678421.2016.1170149 [DOI] [PubMed] [Google Scholar]
- 10.Vignaux L, Vargas F, Roeseler J, et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med 2009;35:840-6. 10.1007/s00134-009-1416-5 [DOI] [PubMed] [Google Scholar]
- 11.Bergbom-Engberg I, Haljamäe H. Patient experiences during respirator treatment--reason for intermittent positive-pressure ventilation treatment and patient awareness in the intensive care unit. Crit Care Med 1989;17:22-5. 10.1097/00003246-198901000-00006 [DOI] [PubMed] [Google Scholar]
- 12.Guo YF, Sforza E, Janssens JP. Respiratory patterns during sleep in obesity-hypoventilation patients treated with nocturnal pressure support: a preliminary report. Chest 2007;131:1090-9. 10.1378/chest.06-1705 [DOI] [PubMed] [Google Scholar]
- 13.Crescimanno G, Canino M, Marrone O. Asynchronies and sleep disruption in neuromuscular patients under home noninvasive ventilation. Respir Med 2012;106:1478-85. 10.1016/j.rmed.2012.05.013 [DOI] [PubMed] [Google Scholar]