Abstract
A 50-year-old man with acute myelogenous leukemia underwent allogeneic bone-marrow transplantation (BMT). He presented with severe diarrhoea 86 days post BMT and was diagnosed with graft-versus-host disease (GVHD) based on skin and rectal biopsies. He complained of numbness and weakness in the distal extremities at 114 days after BMT. His symptoms rapidly deteriorated and he required mechanical ventilation for respiratory failure. His clinical course and the findings of a nerve conduction study fulfilled the criteria for diagnosis of Guillain–Barré syndrome (GBS). Sural nerve biopsy revealed active demyelination and infiltration of macrophages and CD8+ T-cells. After three cycles of intravenous immunoglobulin therapy, his symptoms gradually improved, and he could eventually walk unassisted. Although GBS has been known to develop after allogeneic BMT, the pathogenesis remains unclear, and specific treatment regimens have not been well established. Here, we report a case of GBS, caused by an immune-mediated mechanism related to GVHD, which was successfully treated using intravenous immunoglobulin therapy.
Abbreviations: Ara-C, cytarabine; BU, busulfan; HDAC, high-dose cytarabine; IDA, idarubicin; MTX, methotrexate; PSL, prednisolone
Keywords: Acute inflammatory demyelinating polyneuropathy, Allogeneic bone marrow transplantation, Guillain–Barré syndrome, Intravenous immunoglobulin
Highlights
-
•
AIDP occurs in the clinical setting of graft-versus-host-disease.
-
•
Pathological findings confirmed the infiltration of macrophages and CD8+ T cells.
-
•
Three rounds of intravenous immunoglobulin yielded a good prognosis.
1. Introduction
Guillain–Barré syndrome (GBS) is an acute neurological disorder that is characterized by rapid progressive, symmetrical weakness of the extremities. GBS consists of at least 4 subtypes of acute peripheral neuropathy, of which the most common form is acute inflammatory demyelinating polyradiculoneuropathy (AIDP) [1].
Peripheral neuropathy is an uncommon complication of allogeneic bone marrow transplantation (BMT) and 4% of cases develop neuropathy within the first 3 months after BMT [2]. Most peripheral neuropathies after allogeneic BMT are of the AIDP subtype [2], [3], [4], [5], [6], possibly due to the increased susceptibility to infections and defects in both cell-mediated and humoral immunity. AIDP typically occurs in the clinical setting of graft-versus-host-disease (GVHD) where immunologically competent donor T-cells and/or autoantibodies attack host tissues [2]. However, the number of patients and neuropathological evaluations reported to date are too limited to suggest specific regimens for treatment.
Here, we report a patient with GBS whose neurophysiological finding revealed an AIDP pattern and whose pathological findings confirmed infiltration of macrophages and CD8+ T-cells caused by immune-mediated mechanism related to GVHD. He was effectively treated using 3 rounds of intravenous immunoglobulin and had a good prognosis.
2. Materials and methods
2.1. Neuropathology
Right sural nerve biopsy was performed 41 days after the onset of neurological symptoms. The specimen was divided into 2 portions. One portion was fixed in 2.5% glutaraldehyde in 0.125 M cacodylate buffer (pH 7.4) and embedded in epoxy resin for morphometric and ultrastructural studies. The density of myelinated fibres was assessed using toluidine blue staining. Another fraction was processed for a teased-fibre study. The second portion of the specimen was fixed in 10% formalin and paraffin embedded. Following sectioning, the tissue was stained with haematoxylin and eosin and the Kluver–Barrera method. Immunohistochemical studies were performed on consecutive, deparaffinised sections using the following antibodies: mouse monoclonal anti-CD4, anti-CD8, anti-CD68, and anti-CD20 (Dako).
2.2. Literature review
Two independent investigators (YT and YU) searched PubMed on January 20, 2016, using the term “allogeneic bone marrow transplantation”, “hematopoietic stem cell transplantation” AND each of the following terms: “Guillain-Barre syndrome”, “neuropathy” and “neurological complication”. Only papers written in English were included. Additional articles were sourced manually by searching the citations of relevant articles. We included 10 full-text articles that described patients who developed GBS after BMT [3], [4], [5], [6], [7], [8], [9], [10], [11], [12] (Table 1) and 2 clinical review articles [13], [14].
Table 1.
Analysis of the patients who developed GBS after allogeneic BMT.
| Age/gender | Tumor | GVHD (at the time of GBS) | GBS on set after BMT | CMV antigenomia | Treatment | Outcome | Cause |
|---|---|---|---|---|---|---|---|
| 50/male | AML | Chronic GVHD | 114 days | (+) | IVIg | Recovery | GVHD > CMV |
| 64/male | WM | Unclear | (−) | IVIG | Alive | ||
| 59/male | AML | Unclear | (−) | IVIG, rituximab | Not known | ||
| 37/male | AML | Unclear | (−) | IVIG | Alive | ||
| 44/female | MDS | Unclear | (+) | IVIG, rituximab | Alive | ||
| 58/female | NHL | (−) | Steroids, IVIG, plasmapheresis, cyclosporin | Recovery following cyclosporin | Discontinuation of immunosuppressant, GVHD | ||
| 58/male | MDS | Possible chronic GVHD | 69 days | (−) | IVIG, rituximab, steroids | Alive | Possible GVHD |
| 34/female | ALL | Acute GVHD | 78 days | (+) | IVIG, cyclosporin | Recovery | Immune-mediated |
| 40/female | CML | Acute GVHD | Steroids, cyclosporin, IVIG, plasmapheresis | Partial initial improvement but ultimate death | GVHD | ||
| 18–60 | MDS | 142 days | (+) | IVIG | Death following respiratory failure | ||
| 18–60 | NHL | Chronic GVHD | 160 days | (+) | IVIG, rituximab, plasmapheresis | Death following respiratory infection | |
| 18–60 | AML | Possible chronic GVHD | 101 days | (+) | No specific treatment | Death following respiratory failure | |
| 16/male | T cell ALL | 6 days | (−) | IVIG | Death | Ara-C treatment prior to transplantation | |
| 17/male | T cell Lymphoma | 3 days | (−) | IVIG | Death | Ara-C treatment prior to transplantation | |
| 18/male | T cell ALL | 2 days | (−) | IVIG | Death | Ara-C treatment prior to transplantation | |
| 34/female | CML | No GVHD | 120 days | Plasmapheresis | Improvement | ||
| 27/male | HD | No GVHD | 450 days | Plasmapheresis | Recovery | ||
| 34/male | AML | Mild GVHD | 120 days | Plasmapheresis | Improvement | ||
| 59/female | CML | Mild GVHD | 330 days | (−) | IVIG, plasmapheresis | Death | |
| 43/male | CML | Acute GVHD | 163 days | (+) | IVIG, plasmapheresis | Very slight neurological deficiency | HHV-6 |
| 23/male | AML | No GVHD | 42 days | IVIG, plasmapheresis | Death | CMV |
GVHD—graft versus host disease; BMT—bone marrow transplant; CVM—cytomegalovirus; AML—acute myeloid leukemia; CML—chronic myeloid leukemia; MDS—myelodysplastic syndrome; NHL—Non-Hodgkin's Lymphoma; HD—Hodgkin's disease; WM—Waldenstrom macroglobulinemia; ALL—acute lymphoblastic leukemia; IVIG—intravenous immunoglobulin.
3. Case report
A 50-year-old man was diagnosed with acute myelogenous leukemia (FAB M0 with a complex chromosomal anomaly). Following induction chemotherapy (IDA and Ara-C), 3 cycles of consolidation therapy (HDAC), intrathecal chemotherapy (MTX, Ara-C, and PSL) and conditioning chemotherapy (cyclophosphamide and BU), an allogeneic BMT was performed with his HLA-matched older brother acting as donor. As prophylaxis for GVHD, cyclosporine and MTX were administered. On day 68 after BMT, he presented erythema exudative multiforme, followed by severe diarrhoea on day 86, and was diagnosed with acute GVHD (Grade III) based on skin and rectal biopsies. After he was treated with PSL (60 mg; 1 mg/kg bodyweight), his symptoms gradually improved to Grade II, and the dosage of PSL was gradually reduced to 20 mg. The neurological symptoms began on day 114 after BMT where he complained of numbness in his distal upper and lower extremities and difficulty in swallowing. The weakness of his extremities rapidly increased and on day 116 he required mechanical ventilation for respiratory failure. Neurological examination revealed complete tetraplegia and absence of deep tendon reflexes. He showed hyperesthesia in his distal upper and lower extremities.
Serological tests revealed no abnormalities. Although serological markers for Epstein–Barr virus, herpes simplex virus (HSV), varicella-zoster virus (VZV), HIV, mycoplasma, listeria, and Campylobacter jejuni were negative, pp65 cytomegalovirus (CMV) antigenaemia was positive (14/50,000 cells). We repeated tests for CMV antigenaemia once a week; and the tests became negative after the use of ganciclovir for 1 month after the onset of neurological symptoms. His anti-ganglioside antibodies, including GM1IgG, were all negative. His cerebrospinal fluid (CSF) showed a mild increase in cells (19/μl) and an elevation in protein levels (118 mg/dl). Polymerase chain reaction on the CSF was also negative for HSV, VZV, and CMV both at the onset of neurological symptoms and 1 month thereafter. Two days after the onset of neurological symptoms, nerve conduction studies showed delayed motor nerve conduction velocity (left median nerve: 31.7 m/s, ulnar nerve: 41.8 m/s, tibial nerve: 24.8 m/s) with temporal dispersion and decreased amplitude of compound muscle action potential (left median nerve: 420 μV, ulnar nerve: 640 μV, tibial nerve: 230 μV). F waves and sensory nerve action potentials could not be evoked. His cervical and lumber MRI showed no abnormal lesions.
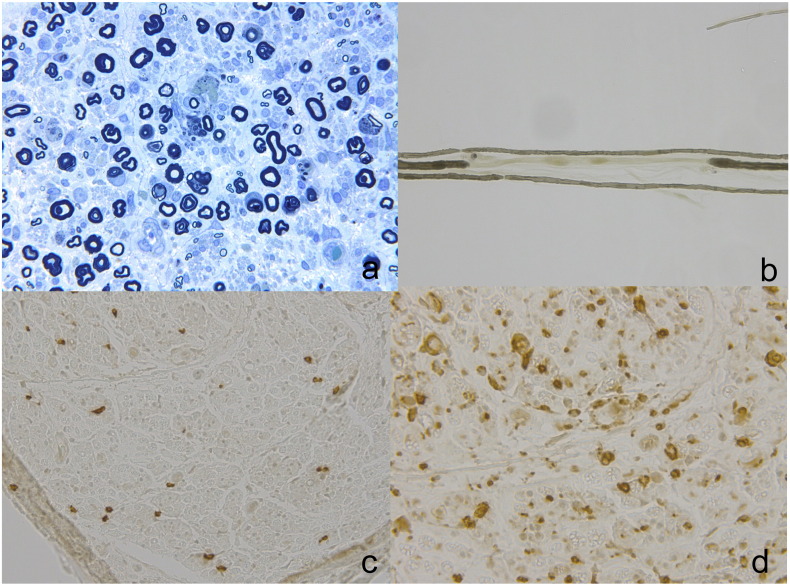
In semi-thin cross sections, the total myelinated fibre densities were moderately decreased (Fig. 1a). Myelin ovoids and endoneurial oedema were observed. There was no evidence of vasculitis or abnormal deposits. Teased-fibre studies showed that the frequency of segmental de/remyelination and axonal degeneration was 30.4% and 15.2%, respectively (Fig. 1b). Scattered lymphocytes and numerous macrophages were detected in the parenchyma. Immunohistochemical studies confirmed infiltration of CD8-positive cytotoxic T-cells and CD68-positive macrophages (Fig. 1c and d). These neurophysiological and pathological findings were compatible with the diagnosis of AIDP.
Fig. 1.
Pathological findings of right sural nerve biopsy.
Toluidine blue stain showing loss of myelinated fibres and myelin ovoids. Large fibres and small fibres were both impaired (a). Teased-fibre preparations revealed marked segmental demyelination (b). Immunostaining showed infiltration of CD8+ T-cells (c) and severe infiltration of CD68+ cells (d).
The patient was treated with 2 courses of intravenous immunoglobulin (IVIG, 400 mg/kg per day) for 5 days; however, his symptoms remained unchanged. As a treatment of GBS, we also considered plasma exchange (PE), given the severity of his motor symptoms. However, since his general status was not good and he presented thrombocytopenia (PLT 27–58 × 103/μl), we decided against PE. Muscle strength gradually improved after the third round of IVIG therapy, and ventilator weaning was possible at 86 days after the onset of neurological symptoms. He was able to walk with assistance 130 days after the onset of neurological symptoms, and eventually regained the ability to walk without assistance.
4. Discussion
We here reported on a male patient who developed severe peripheral neuropathy at the time of recovery from acute GVHD after allogeneic BMT, when immunosuppression therapy was gradually attenuated. Although symptoms of acute GVHD, such as erythema exudative multiforme and diarrhoea had improved, neurological symptoms presented on day 114 after BMT. Based on the diagnostic criteria of acute and chronic GVHD, he was diagnosed with overlap syndrome since the neurological symptoms newly occurred more than 100 days after BMT [15]. Toxins, infection, and critical illness polyneuropathy were excluded as causes in the present case and neurophysiological and neuropathological findings were compatible with a diagnosis of AIDP. He was successfully treated with 3 rounds of IVIG.
Peripheral neuropathy appears to be a rare complication of BMT that can develop as early as 2 days and up to 15 months after BMT [3], [7]. Wen et al. reviewed several studies that reported the presentation of GBS following allogenic BMT and proposed several mechanisms for the pathogenesis of GBS after BMT including the development of an aberrant immunological response to infections, drug toxicity, and a possible manifestation of GVHD [3]. Fujisaki proposed that the mechanism of GBS after allogeneic BMT was not direct neural involvement by CMV and humorally mediated cross-reaction, but rather peripheral expansion of T-cells following CMV infection [5]. We reviewed the recent developments in the field in order to better understand the pathogenesis of GBS following BMT (Table 1).
Previous studies found that 6 of 11 patients were positive for serological markers of Campylobacter jejuni or CMV and 4 of these 6 patients presented with some form of GVHD [3]. One study of 85 patients reported that 3 patients with GBS after BMT, who presented with EBV or CMV infection, 1 of which was diagnosed with GVHD [4]. Peripheral neuropathy has been shown to occur in the clinical course of GVHD. In 1 case, an allogeneic BMT patient developed GBS as the leading manifestation of GVHD subsequent to discontinuation of immunosuppressive medication with cyclosporine; resumption of immunosuppressive medication improved the patient's condition [6]. In the present study, the symptoms developed almost 6 months after chemotherapy and biopsy results indicated GVHD, which was then treated with immunosuppression therapy. Since it occurred during the timing when PSL as a treatment of acute GVHD was reduced, an immune-mediated mechanism related to GVHD seemed plausible. In addition, while the titre was low and there were no symptoms of viral infection, the patient was positive for the CMV antigen and these tests became negative at 1 month after starting treatment with ganciclovir. We cannot totally exclude the involvement of CMV infection as a factor of GBS in the present patient. Therefore, an immune-mediated mechanism related to GVHD, and to some extent, CMV infection, appeared to be the most likely cause of GBS in the present case.
While many histopathological studies have been performed for GBS cases after BMT, only 2 studies have performed immunohistochemical analysis on sural nerve biopsies. In 1 case, an axonal type of GBS was preceded by CMV infection without clinical symptoms of GVHD, where axonal loss and infiltration of plasma cells was detected [4]. In the other case, the axonal type of GBS was preceded by CMV infection after acute GVHD and infiltration of CD8+ T-cells and macrophages were detected [5]. Similarly, in our study, CD8+ T-cell and macrophage infiltration were detected along with active demyelinating patterns and axonal degeneration. Thus, our results suggest that CD8+ T-cell activation may play an important role in the pathophysiology of GBS at the time of recovering from acute GVHD after BMT.
Most patients who develop GBS post-transplant recover after treatment with IVIG and/or PE [3], [8]. Usually, neurological symptoms gradually improve within 1 to several months after these treatments [3], [11]. In 1 report, although muscle strength remained unchanged after IVIG by day 88 after neurological symptom onset, the patient displayed rapid recovery of muscle strength by day 150 [5]. In some cases, the combination of IVIG and rituximab appeared to have a better outcome [8], [9]. Thone et al. have reported 1 case of GBS after BMT who was treated with cyclosporine after a poor response to IVIG and PE [6]. In that case, the patient required artificial ventilation at 18 days after the neurological symptom onset. Following cyclosporine treatment initiation at day 26, his symptoms improved markedly and he was weaned off artificial ventilation by 14 days later. However, in a case report of GBS associated with CMV infection after allogeneic hematopoietic stem cell transplantation and in a review of GBS after allogeneic hematopoietic stem cell transplantation, the outcome of GBS was poor, and all patients (n = 4) died after respiratory and/or multisystem failure 1–5 months after transplant [12], [13]. In the present study, the patient was treated with cyclosporine, MTX, as well as PSL for GVHD. While the GVHD symptoms, such as severe diarrhoea and mild skin rash, improved with this approach, the GBS symptoms persisted. After treatment with repetitive IVIG, his prognosis was good. Since the mechanism of GBS after allogeneic BMT may vary, a combined treatment approach of treating infection and GVHD with IVIG and/or PE seems to be effective. Several rounds of IVIG may be useful for severe forms of GBS after allogeneic BMT.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Hughes R., Cornblath D. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 2.Patchell R.A. Neurological complications of organ transplantations. Ann. Neurol. 1994;36:688–703. doi: 10.1002/ana.410360503. [DOI] [PubMed] [Google Scholar]
- 3.Wen P.Y., Alyea E.P., Simon D. Guillain-Barre syndrome following allogeneic bone marrow transplantation. Neurology. 1997;49:1711–1714. doi: 10.1212/wnl.49.6.1711. [DOI] [PubMed] [Google Scholar]
- 4.Avivi I., Chakrabarti S., Kottaridis P. Neurological complications following alemtuzumab-based reduced-intensity allogeneic transplantation. Bone Marrow Transplant. 2004;34:37–42. doi: 10.1038/sj.bmt.1704538. [DOI] [PubMed] [Google Scholar]
- 5.Fujisaki G., Kami M., Murashige N. Guillain–Barré syndrome associated with rapid immune reconstitution following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:617–619. doi: 10.1038/sj.bmt.1705283. [DOI] [PubMed] [Google Scholar]
- 6.Thone J., Lamprecht S., Hohaus A. Guillain-Barre syndrome as leading manifestation of Graft-versus-Host Disease in an allogenic bone marrow transplanted patient. J. Neurol. Sci. 2010;292:114–116. doi: 10.1016/j.jns.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez V., Kuehnle I., Heslop H.E. Guillain-Barré syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:515–517. doi: 10.1038/sj.bmt.1703412. [DOI] [PubMed] [Google Scholar]
- 8.Delios A.M., Rosenblum M., Jakubowski A.A. Central and peripheral nervous system immune mediated demyelinating disease after allogeneic hemopoieticstem cell transplantation for hematologic disease. J. Neuro-Oncol. 2012;110:251–256. doi: 10.1007/s11060-012-0962-9. [DOI] [PubMed] [Google Scholar]
- 9.Ostronoff F., Perales M.A., Stubblefield M.D. Rituximab-responsive Guillain-Barré syndrome following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2008;42:71–72. doi: 10.1038/bmt.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasilowska-Adamska B., Lysiak Z., Halaburda K. Guillain-Barre syndrome—pathological connection with GvHD after allogeneic bone marrow transplantation. Ann. Transplant. 2006;11:10–11. [PubMed] [Google Scholar]
- 11.Tomaszewska A., Nasilowska-Adamska B., Dzieciatkowski T. Simultaneous human herpesvirus 6-associated encephalitis and Guillain-Barre syndrome in a patient after matched unrelated donor haematopoietic stem cell transplantation. Arch. Med. Sci. 2010;6:288–290. doi: 10.5114/aoms.2010.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Boluda J.C., Lis M.J., Goterris R. Guillain-Barre syndrome associated with cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2005;7:93–96. doi: 10.1111/j.1399-3062.2005.00098.x. [DOI] [PubMed] [Google Scholar]
- 13.Grauer O., Wolff D., Bertz H. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. 2010;133:2852–2865. doi: 10.1093/brain/awq245. [DOI] [PubMed] [Google Scholar]
- 14.Ruzhansky K.M., Brannagan T.H. Neuromuscular complications of hematopoietic stem cell transplantation. Muscle Nerve. 2015;52:480–487. doi: 10.1002/mus.24724. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich A.H., Weisdorf D., Pavletic S. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]