Abstract
Background
SR-16234 is a selective estrogen receptor modulator (SERM) structurally different from approved SERM and has been reported to have estrogen receptor (ER) α antagonistic activity and strong affinity with a weak partial agonistic activity to ERβ receptor. SR-16234 showed strong inhibitory effects on transplanted endometrial cysts in the endometriosis model of rat and mouse. In this clinical trial, efficacy and safety of SR-16234 have been evaluated in endometriosis patients.
Methods
This trial was an open-label single arm clinical trial. Ten patients with dysmenorrhea and pelvic pain associated with endometriosis and adenomyosis were enrolled in this trial, and received 40 mg of SR-16234 once daily for 12 weeks. The primary endpoint was the visual analogue scale (VAS) of pelvic pain. The secondary endpoints included dysmenorrhea score, pelvic pain score, objective observations (stiffness of Douglas’ pouch, limitation of uterine movement, size of ovarian chocolate cysts, thickness of endometrium, and serum CA125 concentration) and safety.
Results
After oral administration of SR-16234 40 mg for 12 weeks, there were statistically significant decreases in pelvic pain VAS, total pelvic pain score, total dysmenorrhea score, stiffness of Douglas’ pouch, limitation of uterine movement compared with the baseline values.
Conclusion
The present trial suggested that a selective estrogen receptor modulator could be used for treatment of pain associated with endometriosis for the first time.
Keywords: estrogen receptor, endometriosis, selective estrogen receptor modulator, pelvic pain, open clinical trial
Endometriosis is a chronic and recurrent gynecological disease affecting 10% of reproductive age women. As it has been considered as an estrogen dependent disease, modalities to suppress endogenous estrogen levels have been widely utilized. Gonadotropin releasing hormone (GnRH) agonist has been a representative agent which can induce hypoestrogenic state and reduce pain symptoms and endometriosis lesions.1 In addition, efficacy of aromatase inhibitors has been reported and is now used as an off label indication in menopausal patients.2, 3
Estrogen receptor antagonist or so called SERM including tamoxifen and fluvestrant have already established clinical efficacy for estrogen receptor positive breast cancer. Several SERMs have been evaluated in animal models of endometriosis, including raloxifene,4, 5 LY-2066948,6 TZE-53237 and bazedoxifene,8 and efficacies on the regression of endometriotic lesions have been reported. So far, however, efficacy of SERM has not been reported in endometriosis patients.
Tamoxifen, the first generation of SERM for treatment of breast cancer, induced endometriosis in post-menopausal breast cancer patients.9, 10 Raloxifene used for postoperative patients with endometriosis showed a significantly shortened time for the return of chronic pelvic pain.11 Partial agonistic activity of those SERMs to ERα is suggested as a potential reason to be detrimental for endometriosis.
SR-16234 is a SERM structurally different from the SERMs mentioned above and has been reported to have ERα antagonistic activity and strong affinity with a weak partial agonistic activity to ERβ receptor.12, 13 Efficacy of this compound for breast cancer has been evaluated.14, 15 We have confirmed inhibitory effects on transplanted endometrial cysts in the endometriosis model of rat and mouse (unpublished data). In this clinical trial, efficacy and safety of SR-16234 have been evaluated in endometriosis patients.
MATERIALS AND METHODS
Patient Selection
Patient inclusion criteria included women of 20 years and older with regular menstrual cycle who have symptomatic endometriosis or adenomyosis. Usage of progestins, oral contraceptives, GnRH agonists, testosterone derivatives, FSH antagonist, aromatase inhibitors, and other drugs that might effect the secretion of sex hormones, and fixation with alcohol through the vagina, laparotomy, laparoscopic therapy or examinations were prohibited throughout the 12 weeks of the period from initiation to completion of SR-16234 administration. All patients provided written, informed consent prior to trial initiation.
Study Design
This was an open-label trial in 10 patients with endometriosis performed at two investigational sites (a university hospital, a general hospital) in Japan. Patients received SR-16234 40mg once daily for 12 weeks, and then follow-up was performed for 4 weeks. Treatment began on the second day of the menstrual cycle. SR-16234 was prepared as a 40mg capsule. The use of analgesic agents was allowed, but other hormonal treatments for pain or vaginal bleeding were prohibited.
The primary endpoint was the VAS of pelvic pain, the secondary endpoints included dysmenorrhea score, pelvic pain score, objective observations (stiffness of Douglas’ pouch, limitation of uterine movement, size of ovarian chocolate cysts, and thickness of endometrium) and safety.
The final protocol was approved by the ethics review committees of each hospital. This clinical trial was registered with the UMIN Clinical Trials Registry (UMIN000019193), and was conducted in accordance with the ethical principles established in the Declaration of Helsinki and consistent with Ethical Guidelines for Clinical Research.
Patient Monitoring
During the 6 weeks of screening period before initiating administration, each patient underwent a pre-recruitment evaluation, consisting of a general medical and gynecologic history, physical and pelvic examination, clinical evaluation of signs and symptoms, VAS of pelvic pain, pelvic pain score (Table 1), dysmenorrhea score (Table 2), use of analgesics, objective observations (stiffness of Douglas’ pouch, limitation of uterine movement (Table 3), size of ovarian chocolate cysts, and thickness of endometrium), clinical laboratory assessments, vital signs and 12 lead ECG. Laboratory assessments include hematology test (white blood cells, platelet, red blood cells, hemoglobin, and hematocrit), biochemical test (total protein, albumin, GOT, GPT, r-GTP, Al-P, LDH, total bilirubin, total cholesterol, high density lipoprotein-cholesterol, TG, BUN, Creatinine, Ca, Fe, P, Na, K, and Cl) and urinalysis. At this period, eligibility for enrollment into the trial was determined, and SR-16234 was prescribed. VAS of pelvic pain, pelvic pain score, dysmenorrhea score, use of analgesics, objective observations (stiffness of Douglas’ pouch, limitation of uterine movement, size of ovarian chocolate cysts, and thickness of endometrium) were evaluated at 4, 8 and 12 weeks. Safety assessments were performed throughout the trial.
Table 1.
Pelvic pain score: Grading and scoring of symptoms and requirement of analgesics
| Grade | Symptom | Score | |
| Pelvic pain | No | None | 0 |
| Slight | Some disturbances to daily work (school work/house keeping) | 1 | |
| Moderate | Disturbances to daily work (school work/house keeping) or need rest in bed | 2 | |
| Severe | Staying in bed unable to work/to do house keeping 3 Use of analgesic No No usage of analgesics | 3 | |
| Use of analgesic | No | No usage of analgesics | 0 |
| Slight | Usage of analgesics in one day due to pelvic pain other than during menstruation from the time of menstruation one month previous | 1 | |
| Moderate | Usage of analgesics for 2–6 days due to pelvic pain other than during menstruation from the time of menstruation of one month previous | 2 | |
| Severe | Usage of analgesics for more than 7 days due to pelvic pain other than during menstruation from the time of menstruation one month previous | 3 |
Total pelvic pain score is the sum of 1) pelvic pain score and 2) use of analgesic score.
Table 2.
Dysmenorrhea score: Grading and scoring of symptoms and requirement of analgesics
| Grade | Symptom | Score | |
| Dysmenorrhea | No | None | 0 |
| Slight | Some disturbances to daily work (school work/house keeping) | 1 | |
| Moderate | Disturbances to daily work (school work/house keeping) or need rest in bed | 2 | |
| Severe | Staying in bed unable to work/to do house keeping 3 Use of analgesic No No usage of analgesics | 3 | |
| Use of analgesic | No | No usage of analgesics | 0 |
| Slight | Usage of analgesics in one day at menstruation | 1 | |
| Moderate | Usage of analgesics for 2–6 days at menstruation | 2 | |
| Severe | Usage of analgesics for more than 7 days at menstruation | 3 |
Total dysmenorrhea score is the sum of 1) dysmenorrhea score and 2) use of analgesic score.
Table 3.
Evaluation method of stiffness of Douglas’ pouch and limitation of uterine movement
| Severity | ||
| Stiffness of Douglas’ pouch | 0 | None: no finding |
| 1 | Very slight: the degree to which the induration is smaller than the size of the little finger tip | |
| 2 | Slight: the degree to which the induration is about the same size as the little finger tip | |
| 3 | Moderate: intermediate degree between “Slight” and “Severe” | |
| 4 | Severe: complete closure of Douglas’ pouch and no resilience | |
| Limitation of uterine movement | 0 | None: no finding |
| 1 | Very slight: the degree to which there is a slight, hardly recognizable limitation of uterine flexibility | |
| 2 | Slight: the degree to which there is a clear limitation of uterine flexibility | |
| 3 | Moderate: the degree to which there is a definite, strong, yet not complete limitation of uterine flexibility | |
| 4 | Severe: complete limitation of uterine movement flexibility |
Statistical Analysis
The mean changes of the VAS of pelvic pain, total pelvic pain score, total dysmenorrhea score, pelvic pain score, dysmenorrhea score, score of analgesic use for pelvic pain, score of analgesic use for dysmenorrhea, stiffness of Douglas’ pouch, limitation of uterine flexibility and thickness of endometrium from baseline were evaluated at 4, 8, 12 weeks after administration of SR-16234.
The changes of the outcome variables were analyzed with the paired t-test. All of the statistical tests were 2-sided with an α level of 0.05 and were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Ten patients were enrolled in this clinical trial and all patients completed the trial until follow-up period. The demographic data of those patients are summarized in Table 4.
Table 4.
Demographic data of the patients
| Number of patients | ||
| Endometriosis | 9 | |
| Adenomyosis | 1 | |
| Age (Years) | ||
| Average | 39.6 | |
| SD | 3.66 | |
| Minimum | 34 | |
| Maximum | 46 | |
| Age of the first menstruation (Years) | ||
| Average | 12.9 | |
| SD | 3.7 | |
| Minimum | 11 | |
| Maximum | 23 | |
| Present menstruation | ||
| Yes | 10 (100%) | |
| Menstruation cycle | ||
| Normal | 10 (100%) | |
| Menstrual cycle (Days) | ||
| Average | 29.4 | |
| SD | 4.14 | |
| Duration of menstruation (Days) | ||
| Average | 6.5 | |
| SD | 1.18 | |
| Volume of menstruation | ||
| Small | 0 | |
| Moderate | 3 (30%) | |
| Large | 7 (70%) | |
| Number of pregnancy | ||
| 0 | 2 (20%) | |
| 1 | 2 (20%) | |
| 2 | 5 (50%) | |
| 3 | 1 (10%) | |
| Number of delivery | ||
| 0 | 4 (40%) | |
| 1 | 1 (10%) | |
| 2 | 5 (50%) | |
| Method of diagnosis | ||
| Histological | 0 | |
| Clinical | 10 (100%) | |
| Surgery | ||
| Yes | 1 (10%) | |
| No | 9 (90%) | |
| Prior therapy | ||
| Yes | 5 (50%) | |
| No | 5 (50%) | |
| Complication | ||
| Yes | 1 (10%) | |
| No | 9 (90%) | |
Changes in pelvic pain VAS
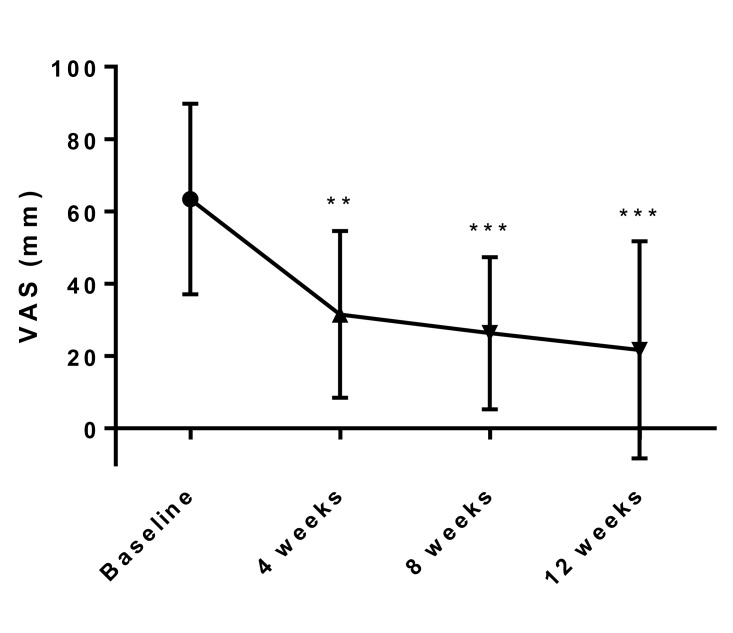
Changes in the mean pelvic pain VAS as the primary endpoint at baseline and 4, 8, 12 weeks after initiation of treatment of SR-16234 are shown in Fig. 1. VAS of pelvic pain significantly decreased at 4, 8 and 12 weeks compared with the baseline.
Fig. 1.
Effects of SR-16234 on pelvic pain VAS in endometriosis patients. Differences in the changes of VAS were analyzed with the paired t-test. Data are represented as mean ± standard deviation. (n = 10, ** P < 0.01, ***P < 0.001)
VAS, visual analogue scale.
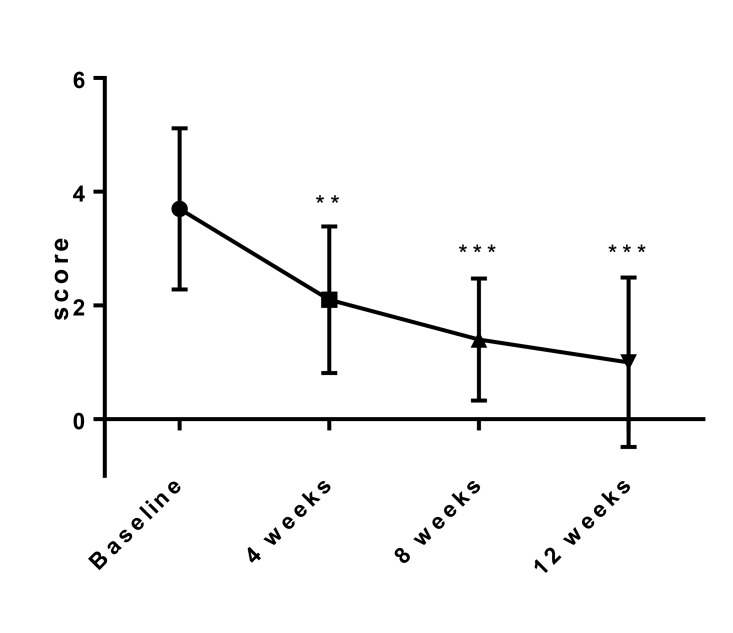
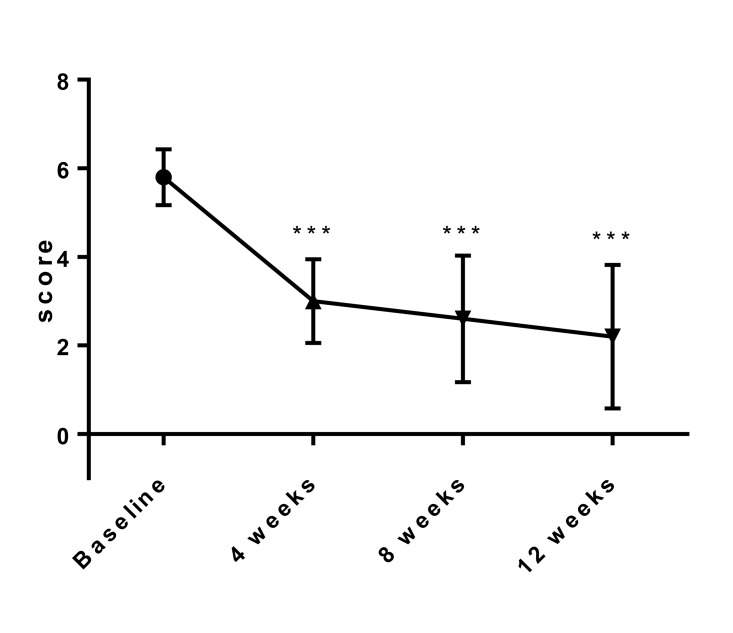
Changes in total pelvic pain score and total dysmenorrhea score
Changes in the mean total pelvic pain score and total dysmenorrhea score at baseline and 4, 8, 12 weeks after initiation of treatment of SR-16234 are shown in Fig. 2, 3, respectively. Total pelvic pain score and total dysmenorrhea score significantly decreased throughout the treatment period compared with the baseline. Pelvic pain score, score of analgesic use for pelvic pain, total dysmenorrhea score and score of analgesic use for dysmenorrhea that consist of total score also significantly decreased throughout the treatment period compared with the baseline (Data not shown).
Fig. 2.
Effects of SR-16234 on total pelvic pain score in endometriosis patients. Differences in the changes of score were analyzed with the paired t-test. Data are represented as mean ± standard deviation. (n = 10, ** P < 0.01, *** P < 0.001)
Fig. 3.
Effects of SR-16234 on total dysmenorrhea score in endometriosis patients. Differences in the changes of score were analyzed with the paired t-test. Data are represented as mean ± standard deviation. (n = 10, *** P < 0.001)
Effects of SR-16234 on stiffness of Douglas’ pouch, limitation of uterine movement, endometrium thickness, and size of chocolate cyst
Douglas’ pouch stiffness and limitation of uterine movement were analyzed in nine patients, and the mean severity of Douglas’ pouch stiffness and limitation of uterine movement decreased after treatment. (3.44 and 3.44 at baseline, 2.44 and 2.55 at 12 weeks, respectively). Endometrial thickness was evaluated in 8 patients, and mean endometrial thickness at 12 weeks (6.87 mm) was less than that of baseline (9.75 mm). Effect of SR-16234 administration on the size of chocolate cyst was evaluated in one patient. A chocolate cyst with volume of 13,305 mm3 was decreased to 4,521 mm3 at 4 weeks and 1,914 mm3 at 8 weeks, respectively and disappeared at 12 weeks.
Adverse events
Three patients reported adverse events in this clinical trial including contusion of the right leg and hemorrhage subcutaneous of the right leg (1), nausea (1) and common wart (1) but none of these conditions were found to have any relation to SR-16234 usage. No serious adverse events were reported in this trial. There were no clinically significant changes in clinical laboratory assessments (hematology, biochemical and urinalysis), vital signs and 12 lead ECG (data not shown).
DISCUSSION
Endometriosis is often manifested by pain symptoms, such as dysmenorrhea, pelvic pain, and dyspareunia. Current therapeutic options include conservative surgery and medical treatment with oral contraceptives, GnRH agonist, danazol, and progestins.16–18
Surgical therapy for endometriosis continues to be the primary therapeutic measure and a Cochrane meta-analysis of 5 randomized controlled studies evaluating laparoscopic treatment of endometriosis compared with diagnostic laparoscopy without treatment reported that pain was significantly improved in the treatment group. However, recurrence rate after surgical therapy is high and may be associated with significant complication rates. In addition, in the case of ovarian endometriosis, there is concern about the risk of ovarian damage and impaired ovarian reserve.19
Medical treatment of pain associated with endometriosis is generally effective with little difference in efficacy observed among the different types of agents used; however, the adverse event profiles of the various drug regimens markedly differ.1, 3, 20, 21
As endometriosis is considered an estrogen dependent disease, induction of a hypoestrogenic condition is one major active mechanism using established medical agents. GnRH agonist is one representative inducer of the hypoestrogenic condition. Although strong efficacy of GnRH agonist is well known for controlling pain symptoms of endometriosis, hypoestrogenic side effects including hot flushes, vaginal dryness, emotional lability, loss of libido, and significant loss in bone mineral density (BMD) are problematic. Reduction of BMD may not be recovered until a few years after completion of the treatment. Due to these concerns, administration period of GnRH agonist is basically limited to less than 6 months.20, 21
Endometriotic implants express a high level of aromatase and generate their own estrogen, which can maintain their viability and growth.22 As aromatase inhibitors inhibit local estrogen production in endometriotic implants, its efficacy for endometriosis has been evaluated.23 A systematic review of eight studies showed that aromatase inhibitors combined with progestogens, oral contraceptives, or GnRH agonists had reduced mean pain scores and lesion size and improved QOL. However, monotherapy with aromatase inhibitor administered to reproductive age women will cause increased FSH levels and subsequent superovulation which may result in ovarian cyst development due to the initial FSH rise.24 Other concerns about prolonged aromatase inhibitor therapy are many, including associated bone loss secondary to hypoestrogenism.24
Due to the estrogen dependent nature of endometriosis, SERMs have been proposed for the treatment of endometriosis.25 However, no SERMs have been reported to be effective in the treatment of endometriosis.3 SERM is an acronymic term for a group of drugs that selectively modulate estrogen receptor. Tamoxifen is a first generation SERM used for the treatment of breast cancer. Usage of tamoxifen as an alternative modality in the treatment of endometriosis, especially for women desiring to conceive, was expected. However, after wide spread use of tamoxifen for breast cancer, occurrences of endometriosis was reported in postmenopausal patients who had been taking tamoxifen for treatment of breast cancer. And it had been suggested that long term tamoxifen users are more likely to have endometrial hyperplasia, endometrial polyps, and/or endometrial cancer.9, 10
As these effects of tamoxifen were considered to be derived from its estrogen receptor agonistic activity, other SERMs that have more selective estrogenic activity were evaluated. Raloxifene has been used for the treatment of postmenopausal osteoporosis. In a randomized clinical trial in biopsy proven endometriosis with chronic pelvic pain, the raloxifene group experienced significant pain and had secondary surgery statistically significantly sooner than the placebo group. This truncated trial concluded that raloxifene statistically significantly shortened the time of return to chronic pelvic pain.11
The mechanism of failure of raloxifene was not well explained in that publication. It may depend on the agonistic activity of raloxifene to the G protein coupled estrogen receptor (GPR30). While many effects of estrogen are mediated by its action at its nuclear estrogen receptors, ERα and ERβ, novel estrogen receptor GPR30 and peripheral administration of GPR30 agonists or estradiol (E2) produces mechanical hyperalgesia within minutes after injection.26 Locally injected raloxifene close to the endometrial implant in the rat model of endometriosis also induced mechanical hyperalgesia at a comparable dose with E2.4
Bazedoxifene reduced the size of endometrial lesions with experimental evidence of an antiproliferative effect in mouse and rat models.8 In addition bazedoxifene was shown to decrease proliferating cell nuclear antigen and estrogen receptor expression in the endometrium of treated animals compared with controls. However, the effectiveness of bazedoxifene on endometriosis in humans has not been published.
The data of the present clinical trial suggests that SR-16234 may alleviate dysmenorrhea and pelvic pain of endometriosis at 40 mg daily dosage by oral administration. As no other SERMs have shown such clinical efficacies in endometriosis, SR-16234 may be the first SERM with reported efficacy for this disease condition. The mechanism action of SR-16234 for endometriosis is not well clarified. Other SERMs have some levels of partial agonistic activity to ERα and that may be the reason for resistance occurring in treatment for breast cancer and could be effective in increasing bone mineral density in osteoporosis patients. Compared with 1st or 2nd generation SERMs including tamoxifene, raloxifene or baldoxifene, SR-16234 seems to be a purer ERα antagonist and that may be one of the reasons it is effective for endometriosis related pain. Its strong affinity to both ERα and ERβ might be important. Bulun et al suggest that deficient methylation of the ERβ promotor results in pathological overexpression of ERβ in endometriotic stromal cells, high levels of ERβ suppress ERα expression and a high ERβ to ERα ratio in endometriotic stromal cells is associated with suppressed progesterone receptor and increased cyclooxygenase-2 levels contributing to progesterone resistance and inflammation.23
As the affinity of SR-16234 to ERβ is 10 to 100 times stronger than other SERMs that have been used for treatment of breast cancer or osteoporosis. In endometriotic tissues where high estrogen content and high ERβ expression, SR-16234 may induce ERβ activation to inhibit growth of endometriotic tissue, inflammatory cytokines, COXs expression, and ERβ receptor resistance.
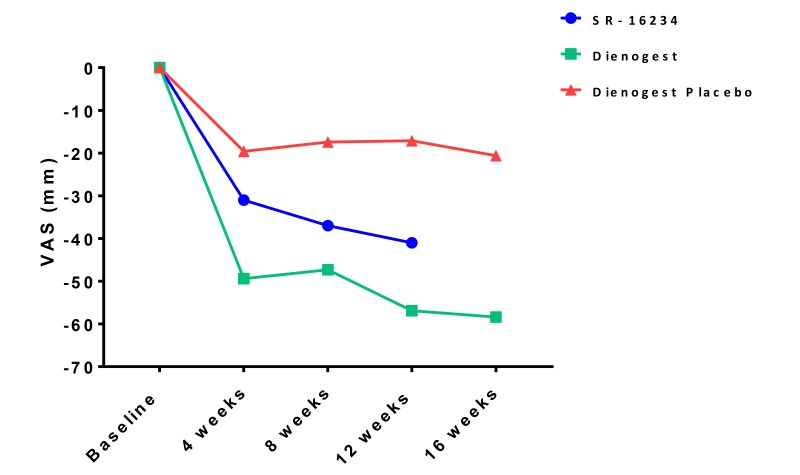
In terms of efficacy of drugs for pain associated with endometriosis, we compared changes in pelvic pain score of SR-16234 and dienogest (data for dienogest and placebo derived from the CTD of dienogest) (Fig. 4). SR-16234 had stronger effects than the placebo, but changes in pelvic pain VAS was more profound with dienogest, suggesting potential utility of SR-16234 in pain management of endometriosis. Further large scale and placebo compared clinical trials in the future are necessary for confirmation.
Fig. 4.
Effects of SR-16234 and dienogest on pelvic pain score (VAS) in endometriosis patients. Data of dienogest and placebo were derived from common technical document (CTD).
Acknowledgments
Acknowledgments: his trial was funded by Nobelpharma Co., Ltd., which provided SR-16234 without charge. The author would like to acknowledge the members of the Advanced Medicine, Innovation and Clinical Research Center, Tottori University Hospital for coordinating the trial. The authors also would like to thank the patients who participated in this trial.
Conflicts of Interest: Dr. Harada reports grants and non-financial support from Nobelpharma Co., Ltd. while conducting this study, grants and non-financial support from Nobelpharma Co., Ltd. outside the submitted work.
REFERENCES
- 1. Harada T. Dysmenorrhea and endometriosis in young women. Yonago Acta Med. 2013;56:81-4. [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal SK, Foster WG. Reduction in endometrioma size with three months of aromatase inhibition and progestin add-back. Biomed Res Int. 2015;2015:1-4. doi: 10.1155/2015/878517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedaiwy MA, Alfaraj S, Yong P, Casper R. New developments in the medical treatment of endometriosis. Fertil Steril. 2017;107:555-65. doi: 10.1016/j.fertnstert.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 4. Yao Z, Shen X, Capodanno I, et al. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg. 2005;18:177-83. doi: 10.1080/08941930591004412 [DOI] [PubMed] [Google Scholar]
- 5. Altintas D, Kokcu A, Kandemir B, Tosun M, Cetinkaya MB. Comparison of the effects of raloxifene and anastrozole on experimental endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:84-7. doi: 10.1016/j.ejogrb.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 6. Geiser AG, Hummel CW, Draper MW, et al. A new selective estrogen receptor modulator with potent uterine antagonist activity, agonist activity in bone, and minimal ovarian stimulation. Endocrinology. 2005;146:4524-35. doi: 10.1210/en.2005-0024 [DOI] [PubMed] [Google Scholar]
- 7. Saito T, Yoshizawa M, Yamauchi Y, et al. Effects of the novel orally active antiestrogen TZE-5323 on experimental endometriosis. Arzneimittelforschung. 2003;53:507-14. doi: 10.1055/s-0031-1297141 [DOI] [PubMed] [Google Scholar]
- 8. Kulak J, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152:3226-32. doi: 10.1210/en.2010-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford MR, Turner MJ, Wood C, Soutter WP. Endometriosis developing during tamoxifen therapy. Am J Obstet Gynecol. 1988;158:1119. [DOI] [PubMed] [Google Scholar]
- 10. Le Bouëdec G, Kauffmann P, Pingeon JM, de Latour M, Lemesle P, Dauplat J, et al. [Post-menopausal endometriosis developed during tamoxifen treatment]. Rev Fr Gynecol Obstet. 1991;86:407-10. [PubMed] [Google Scholar]
- 11. Stratton P, Sinaii N, Segars J, et al. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:88-96. doi: 10.1097/01.AOG.0000297307.35024.b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto Y, Wada O, Takada I, et al. Both N- and C-terminal transactivation functions of DNA-bound ERalpha are blocked by a novel synthetic estrogen ligand. Biochem Biophys Res Commun. 2003;312:656-62. doi: 10.1016/j.bbrc.2003.10.178 [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto Y, Shibata J, Yonekura K, et al. TAS-108, a novel oral steroidal antiestrogenic agent, is a pure antagonist on estrogen receptor alpha and a partial agonist on estrogen receptor beta with low uterotrophic effect. Clin Cancer Res. 2005;11:315-22. [PubMed] [Google Scholar]
- 14. Buzdar AU. TAS-108: a novel steroidal antiestrogen. Clin Cancer Res. 2005;11(2 Pt 2):906s-8s. [PubMed] [Google Scholar]
- 15. Inaji H, Iwata H, Nakayama T, et al. Randomized phase II study of three doses of oral TAS-108 in postmenopausal patients with metastatic breast cancer. Cancer Sci. 2012;103:1708-13. doi: 10.1111/j.1349-7006.2012.02354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Practice Committee of the American Society for Reproductive Medicine Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927-35. doi: 10.1016/j.fertnstert.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 17. Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90:1583-8. doi: 10.1016/j.fertnstert.2007.08.051 [DOI] [PubMed] [Google Scholar]
- 18. Harada T, Momoeda M, Taketani Y, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis--a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91:675-81. doi: 10.1016/j.fertnstert.2007.12.080 [DOI] [PubMed] [Google Scholar]
- 19. Chaichian S, Kabir A, Mehdizadehkashi A, Rahmani K, Moghimi M, Moazzami B, et al. Comparing the Efficacy of Surgery and Medical Therapy for Pain Management in Endometriosis: A Systematic Review and Meta-analysis. Pain Physician. 2017;20:185-95. [PubMed] [Google Scholar]
- 20. Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400-12. doi: 10.1093/humrep/det457 [DOI] [PubMed] [Google Scholar]
- 21. Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Brown J, ed Cochrane database Syst Rev. 2014;(3):CD009590. doi: 10.1002/14651858.CD009590.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Izawa M, Harada T, Taniguchi F, Ohama Y, Takenaka Y, Terakawa N. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertil Steril. 2008;89(5 Suppl):1390-6. doi: 10.1016/j.fertnstert.2007.03.078 [DOI] [PubMed] [Google Scholar]
- 23. Bulun SE, Monsivais D, Kakinuma T, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33:220-4. doi: 10.1055/s-0035-1554053 [DOI] [PubMed] [Google Scholar]
- 24. Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115:818-22. doi: 10.1111/j.1471-0528.2008.01740.x [DOI] [PubMed] [Google Scholar]
- 25. Simsa P, Mihalyi A, Kyama CM, Mwenda JM, Fülöp V, D’Hooghe TM, et al. Selective estrogen-receptor modulators and aromatase inhibitors: promising new medical therapies for endometriosis? . Womens Health (Lond Engl). 2007;3:617-28. doi: 10.2217/17455057.3.5.617 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez P, Bogen O, Levine JD. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain. 2014;155:2680-6. doi: 10.1016/j.pain.2014.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]